Method for preparing high-purity superfine silver powder
An ultra-fine silver and high-purity technology, which is applied in the field of preparation of high-purity ultra-fine silver powder, can solve problems such as low purity, uneven product particle size, and difficult control conditions, and achieve high purity, low cost, and fast response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
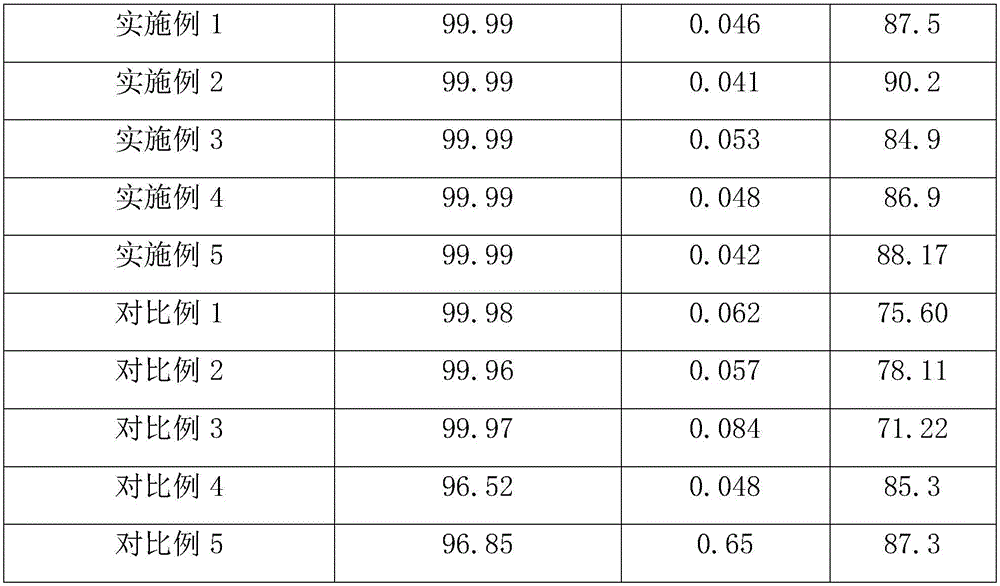
Embodiment 1
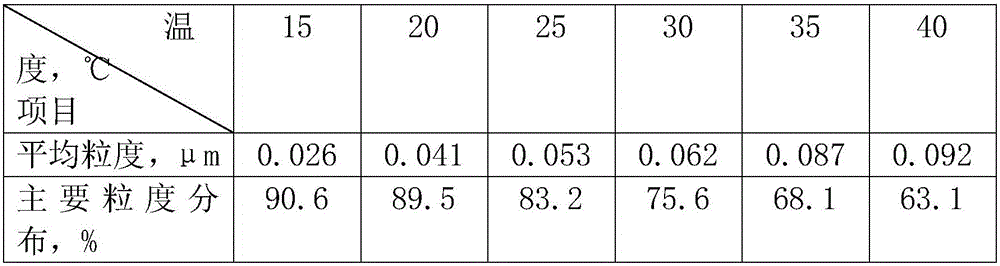
[0023] a. Put 100g of silver nitrate in the first container, add 55ml of deionized water, stir continuously until the silver nitrate is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 14 hours, and filter, Wash and filter the obtained crystals with absolute ethanol to obtain purified silver nitrate crystals;
[0024] b. Put 38g of sodium chloride in the second container, add 105ml of deionized water, stir continuously until the sodium chloride is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 14 hours, and filter , washing and filtering the gained crystals with absolute ethanol to obtain purified sodium chloride crystals;
[0025] c. 85g of silver nitrate crystals purified in step a are placed in the third container, then 105ml of deionized water is added to configure silver nitrate solution, and 30.5g of purified sodium chloride crystals in step b are added to 105ml to remove Ionized wat...
Embodiment 2
[0031] a. Put 100g of silver nitrate in the first container, add 50ml of deionized water, stir continuously until the silver nitrate is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 16 hours, and filter, Wash and filter the obtained crystals with absolute ethanol to obtain purified silver nitrate crystals;
[0032] b. Put 38g of sodium chloride in the second container, add 110ml of deionized water, stir continuously until the sodium chloride is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 16 hours, and filter , washing and filtering the gained crystals with absolute ethanol to obtain purified sodium chloride crystals;
[0033] c, 85g of silver nitrate crystals purified in step a are placed in a third container, then 110ml of deionized water is added to configure silver nitrate solution, and 29.25g of sodium chloride crystals purified in step b are added to 100ml to remove Ionized wate...
Embodiment 3
[0039] a. Put 100g of silver nitrate in the first container, add 60ml of deionized water, stir continuously until the silver nitrate is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 12 hours, and filter, Wash and filter the obtained crystals with absolute ethanol to obtain purified silver nitrate crystals;
[0040] b. Put 38g of sodium chloride in the second container, add 100ml of deionized water, stir continuously until the sodium chloride is completely dissolved, then add absolute ethanol until no crystals are precipitated, age for 12 hours, and filter , washing and filtering the gained crystals with absolute ethanol to obtain purified sodium chloride crystals;
[0041] c. Put 85g of silver nitrate crystals purified in step a in the third container, then add 100ml of deionized water to configure silver nitrate solution, then add 32g of purified sodium chloride crystals in step b to 110ml of deionized Water is configured into so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com