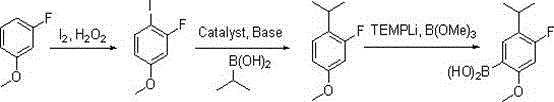Synthesis method of 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid
A technology of methoxybenzene boronic acid and methoxy cumene is applied in the field of pharmaceutical intermediate synthesis, and can solve the problems of large-scale production of unfavorable products, poor reaction selectivity, long synthesis route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
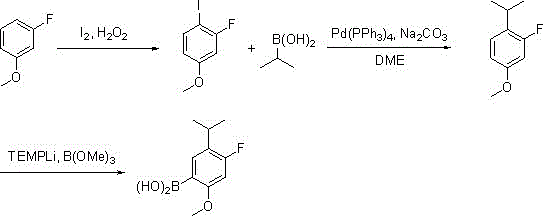
[0025] Synthesis of 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid:
[0026]
[0027] The first step: in the reaction flask, add m-fluoroanisole (12.6 grams, 0.1 moles), 30% hydrogen peroxide (6.2 grams, 0.055 moles) and 130 milliliters of ethanol, after stirring evenly, add iodine (13.2 grams , 0.052 moles), dropwise addition is completed, the reaction solution dissolves clear, and the insulation reaction is continued for 2 hours, the detection reaction is complete, and saturated NaHSO is added 3 The reaction was quenched, 80 milliliters of ethyl acetate was layered, the organic layer was washed with saturated sodium chloride, and distilled to dryness to obtain 22.9 grams of 2-fluoro-4-methoxyiodobenzene;
[0028] Step 2: Mix 2-fluoro-4-methoxyiodobenzene (2.9 g, 91 mmol), isopropylboronic acid (8.0 g, 91 mmol), 1M aqueous sodium carbonate (0.14 mol) and 210 mL of DME After adding the reaction flask, nitrogen gas was blown into the page to remove oxygen, and finally 1...
Embodiment 2
[0031] Synthesis of 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid:
[0032]
[0033] The first step: in the reaction flask, add m-fluoroanisole (12.6 grams, 0.1 moles), 30% hydrogen peroxide (6.2 grams, 0.055 moles) and 130 milliliters of ethanol, after stirring evenly, add iodine (13.2 grams , 0.052 moles), dropwise addition is completed, the reaction solution dissolves clear, and the insulation reaction is continued for 2 hours, the detection reaction is complete, and saturated NaHSO is added 3 The reaction was quenched, 80 milliliters of ethyl acetate was layered, the organic layer was washed with saturated sodium chloride, and distilled to dryness to obtain 22.9 grams of 2-fluoro-4-methoxyiodobenzene;
[0034] Step 2: Mix 2-fluoro-4-methoxyiodobenzene (2.9 g, 91 mmol), isopropylboronic acid (8.0 g, 91 mmol), 1M aqueous potassium carbonate (0.14 mol) and 210 mL of DME After adding the reaction flask, nitrogen gas was blown into the page to remove oxygen, and finall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com