Method for preparing brain natriuretic peptide precursor antigen substitute based on human-derived skeleton protein Fn3
A skeleton protein and brain natriuretic peptide technology, applied in chemical instruments and methods, using vectors to introduce foreign genetic material, expression enhancement stability/folded protein fusion, etc., can solve the problems of small molecules, difficult expression and purification, etc., and achieve high-efficiency expression Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
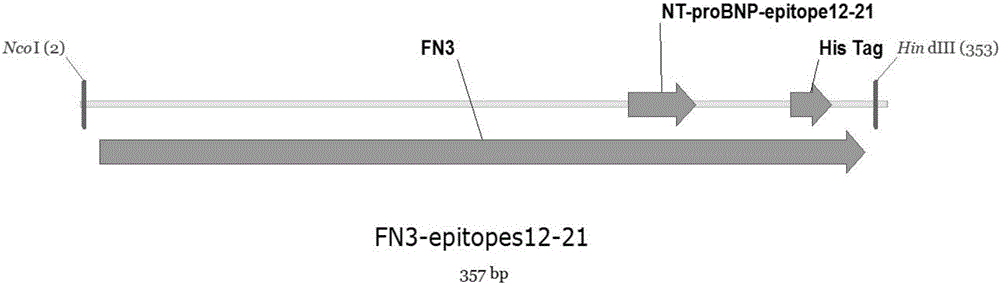
[0023] 1. In this example, human fibronectin type III domain (Fn3) protein is used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues were introduced, and a short peptide sequence encoding NT-proBNP epitope (CTGGAAACCTCGGGCCTGCAGGAACAACGT, encoding 12-21 amino acid residues LETSGLQEQR of NT-proBNP) was introduced into the FGloop region. The fusion gene structure See figure 1 .
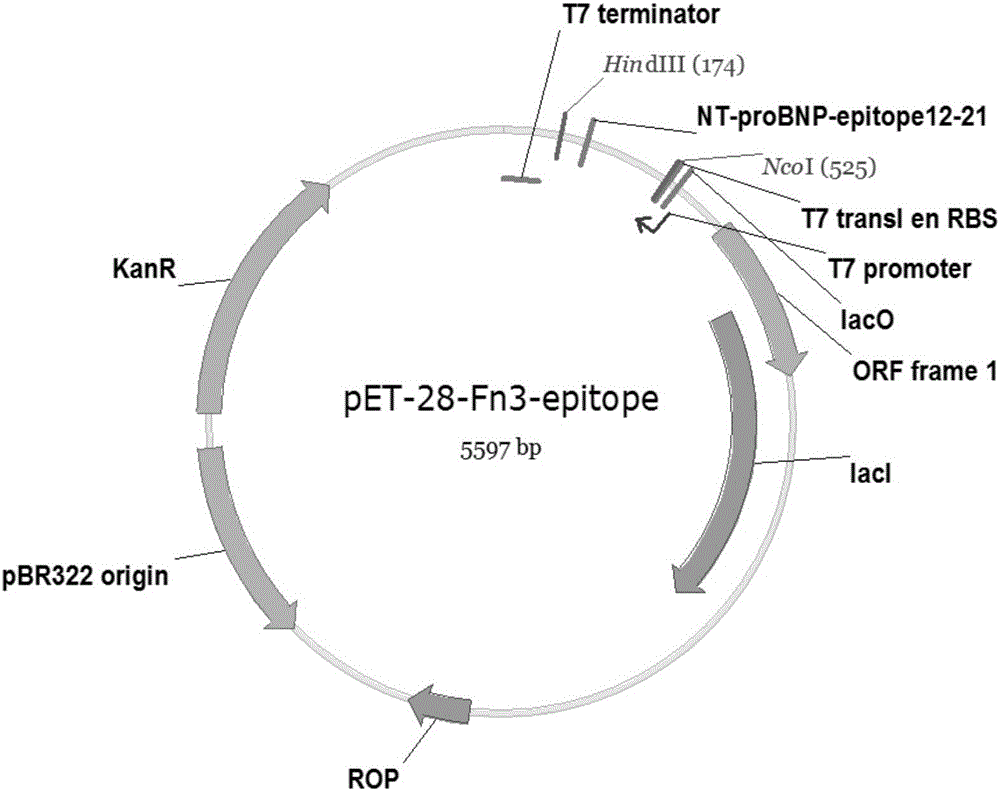
[0024] After the human fibronectin type III domain (Fn3) shown in the sequence table [001] and the NT-proBNP fusion gene expression cassette sequence were synthesized (Nanjing Jinsirui Biotechnology Co., Ltd.), construct Escherichia coli with NcoI and HindIII On the expression vector pET28a(+), the recombinant vector pET28-Fn3-epitope was obtained. For the vector structure, see figure 2 .
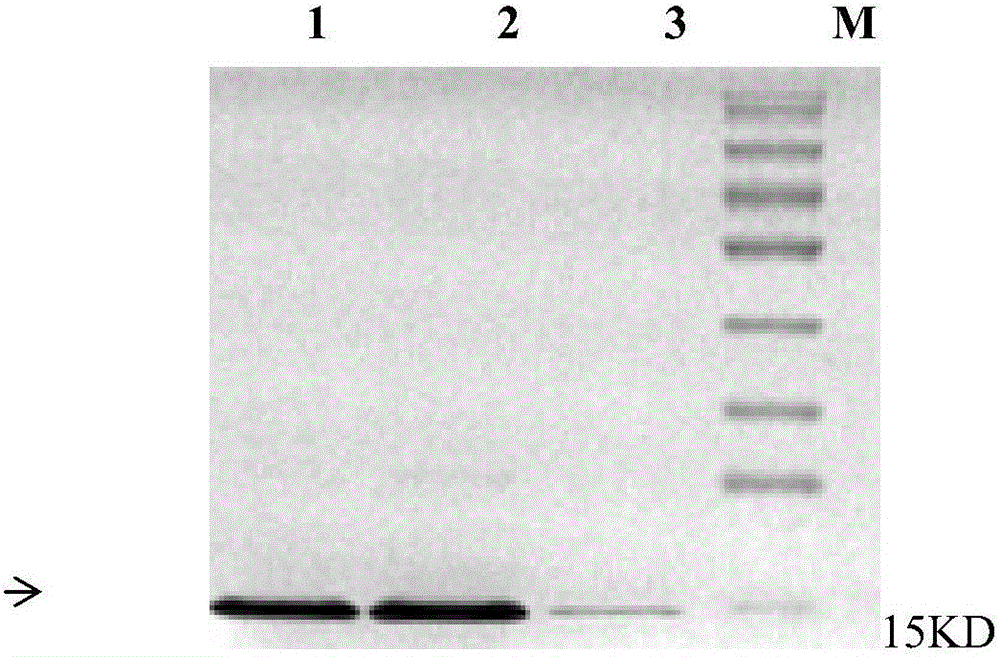
[0025] 2. Electric shock transformation of the constructed expression vector into BL21 (DE3) Escherichia coli strain, and then inoculate the transformants into 10 m...
Embodiment 2
[0029] 1. In this example, human fibronectin type III domain (Fn3) protein is used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues were introduced, and a short peptide sequence encoding NT-proBNP epitope (CTGGAAACCTCGGGCCTGCAGGAACAACGT, encoding 12-21 amino acid residues LETSGLQEQR of NT-proBNP) was introduced into the FGloop region. The fusion gene structure See figure 1 .
[0030] After the human fibronectin type III domain (Fn3) shown in the sequence table [001] and the NT-proBNP fusion gene expression cassette sequence were synthesized (Nanjing Jinsirui Biotechnology Co., Ltd.), construct Escherichia coli with NcoI and HindIII On the expression vector pET28a(+), the recombinant vector pET28-Fn3-epitope was obtained. For the vector structure, see figure 2 .
[0031] 2. Electric shock transformation of the constructed expression vector into BL21 (DE3) Escherichia coli strain, and then inoculate the transformants into 10 m...
Embodiment 3
[0035] 1. In this example, human fibronectin type III domain (Fn3) protein is used as a model gene (EMBL accession number AJ320527). At the 5′ end of the gene, 6 histidine residues were introduced, and a short peptide sequence encoding NT-proBNP epitope (CTGGAAACCTCGGGCCTGCAGGAACAACGT, encoding 12-21 amino acid residues LETSGLQEQR of NT-proBNP) was introduced into the FGloop region. The fusion gene structure See figure 1 .
[0036] After the human fibronectin type III domain (Fn3) shown in the sequence table [001] and the NT-proBNP fusion gene expression cassette sequence were synthesized (Nanjing Jinsirui Biotechnology Co., Ltd.), construct Escherichia coli with NcoI and HindIII On the expression vector pET28a(+), the recombinant vector pET28-Fn3-epitope was obtained. For the vector structure, see figure 2 .
[0037] 2. Transform the Escherichia coli strain BL21 (DE3) by electroporation with the constructed expression vector, then inoculate the transformant into 10 millil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com