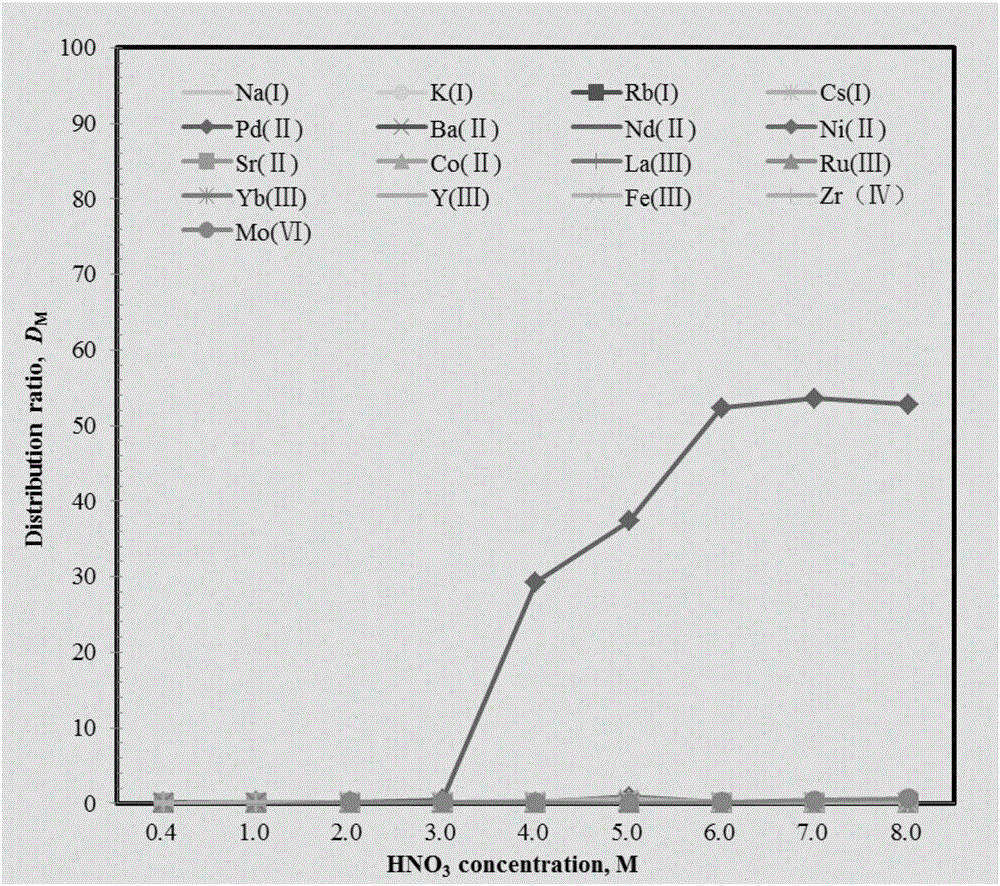
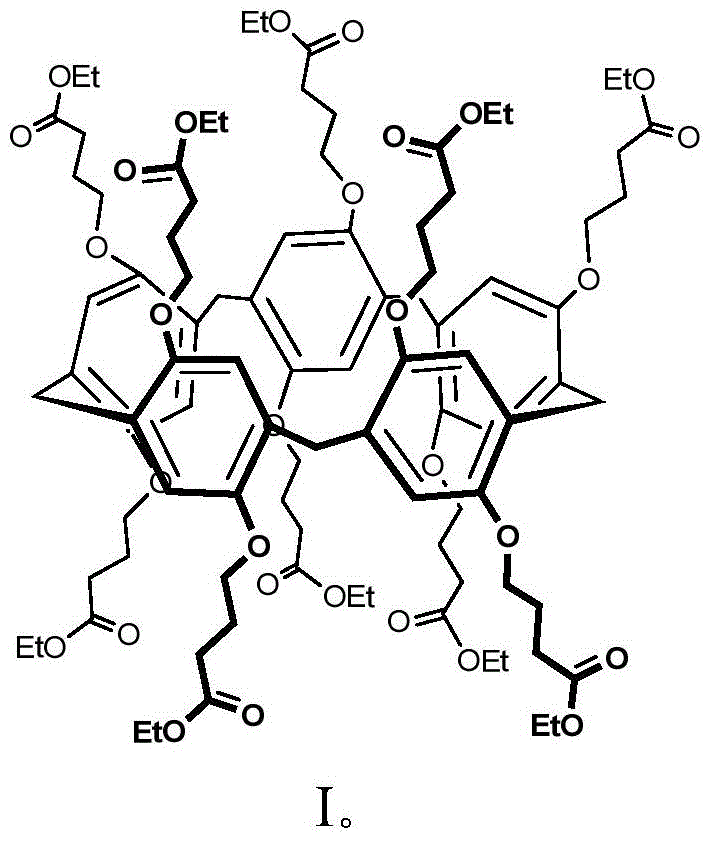
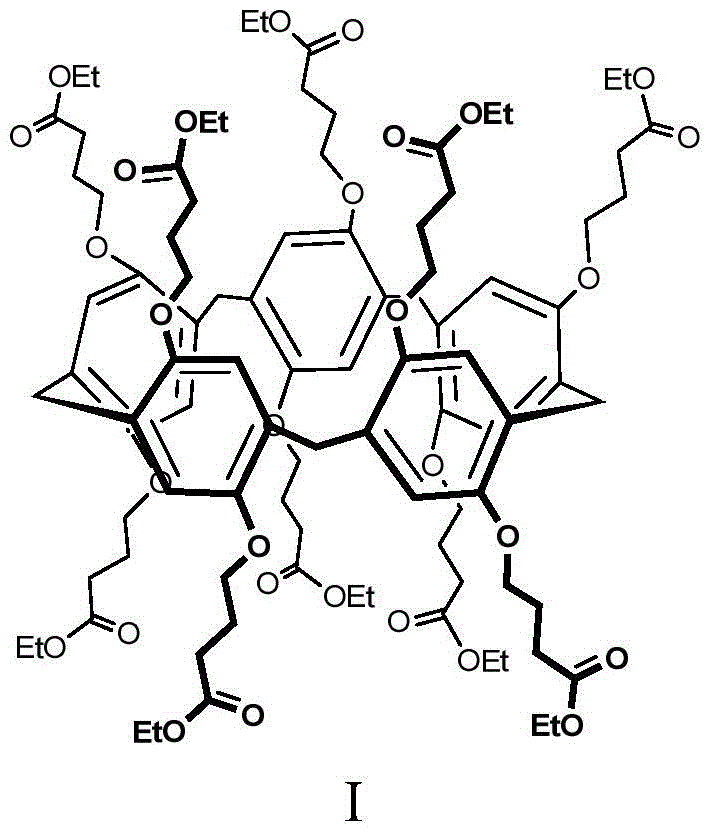
Method for extracting and separating palladium ions through pillar arene ramification
A technology of derivatives and pillar aromatics, which is applied in the field of extracting and separating palladium ions by using pillar aromatic derivatives, can solve the problems of not finding a high-efficiency recovery path, long radioactive cycle, high radioactivity, etc., and achieves easy operation, strong selectivity, The effect of efficient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of pillar arene derivatives, comprising:
[0035] (1) 1,4-p-methoxybenzene and paraformaldehyde are reacted under the conditions of boron trifluoride ether as a catalyst and dry dichloromethane or 1,2-dichloroethane as a solvent to obtain permethoxy Pillar[5]arenes.
[0036] Wherein, the molar ratio of 1,4-p-methoxybenzene and paraformaldehyde is 1:1, the molar ratio of 1,4-p-methoxybenzene and catalyst boron trifluoride ether is 1:1, and the reaction temperature is 28° C., the reaction time is 3 minutes, and the amount of solvent used is 5 ml per mmol of 1,4-p-methoxybenzene.
[0037] (2) Using the permethoxypyrro[5]arene obtained in step (1) as a raw material, react with boron tribromide under the condition of dry chloroform as a solvent to obtain perhydroxypyrro[5]arene.
[0038] Wherein, the molar ratio of permethoxycolumn[5]arene to boron tribromide is 1:20, the reaction temperature is 25°C, the reaction time is 2 days, and the amount of solv...
Embodiment 2
[0043] A preparation method of pillar arene derivatives, comprising:
[0044] (1) 1,4-p-methoxybenzene and paraformaldehyde are reacted under the conditions of boron trifluoride ether as a catalyst and dry dichloromethane or 1,2-dichloroethane as a solvent to obtain permethoxy Pillar[5]arenes.
[0045] Wherein, the molar ratio of 1,4-p-methoxybenzene and paraformaldehyde is 1:2, the molar ratio of 1,4-p-methoxybenzene and catalyst boron trifluoride ether is 1:1.1, and the reaction temperature is 29° C., the reaction time is 3 minutes, and the amount of solvent used is 8 ml per mmol of 1,4-p-methoxybenzene.
[0046] (2) Using the permethoxypyrro[5]arene obtained in step (1) as a raw material, react with boron tribromide under the condition of dry chloroform as a solvent to obtain perhydroxypyrro[5]arene.
[0047] Wherein, the molar ratio of permethoxycolum[5]arene to boron tribromide is 1:22, the reaction temperature is 30°C, the reaction time is 3 days, and the amount of sol...
Embodiment 3
[0051] A preparation method of pillar arene derivatives, comprising:
[0052] (1) 1,4-p-methoxybenzene and paraformaldehyde are reacted under the conditions of boron trifluoride ether as a catalyst and dry dichloromethane or 1,2-dichloroethane as a solvent to obtain permethoxy Pillar[5]arenes.
[0053] Wherein, the molar ratio of 1,4-p-methoxybenzene and paraformaldehyde is 1:4, the molar ratio of 1,4-p-methoxybenzene and catalyst boron trifluoride ether is 1:1.2, and the reaction temperature is 30° C., the reaction time is 3 minutes, and the amount of solvent used is 10 ml per mmol of 1,4-p-methoxybenzene.
[0054] (2) Using the permethoxypyrro[5]arene obtained in step (1) as a raw material, react with boron tribromide under the condition of dry chloroform as a solvent to obtain perhydroxypyrro[5]arene.
[0055] Wherein, the molar ratio of permethoxypyrro[5]arene to boron tribromide is 1:25, the reaction temperature is 35°C, the reaction time is 4 days, and the amount of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com