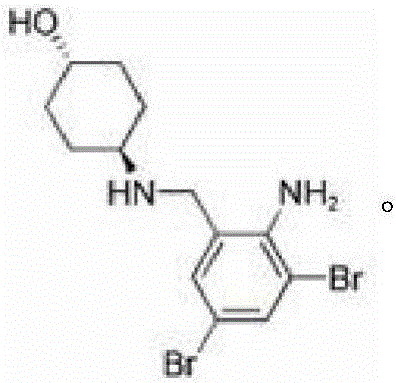
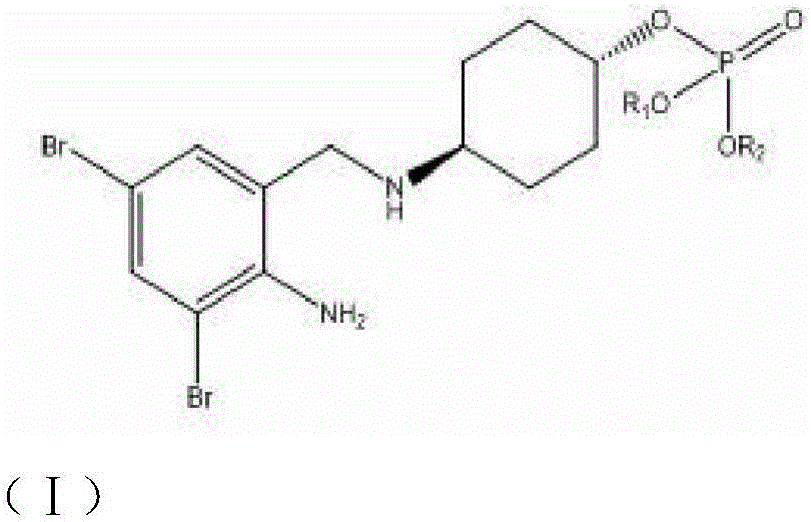
Ambroxol derivative and application
A derivative, ambroxol technology, applied in the field of medicinal chemistry, can solve problems such as incompatibility and incompatibility, and achieve the effects of enhanced drug efficacy, wide pH dissolution range, and improved oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 compound 1
[0038]
[0039] The preparation scheme is shown in the following reaction formula:
[0040]
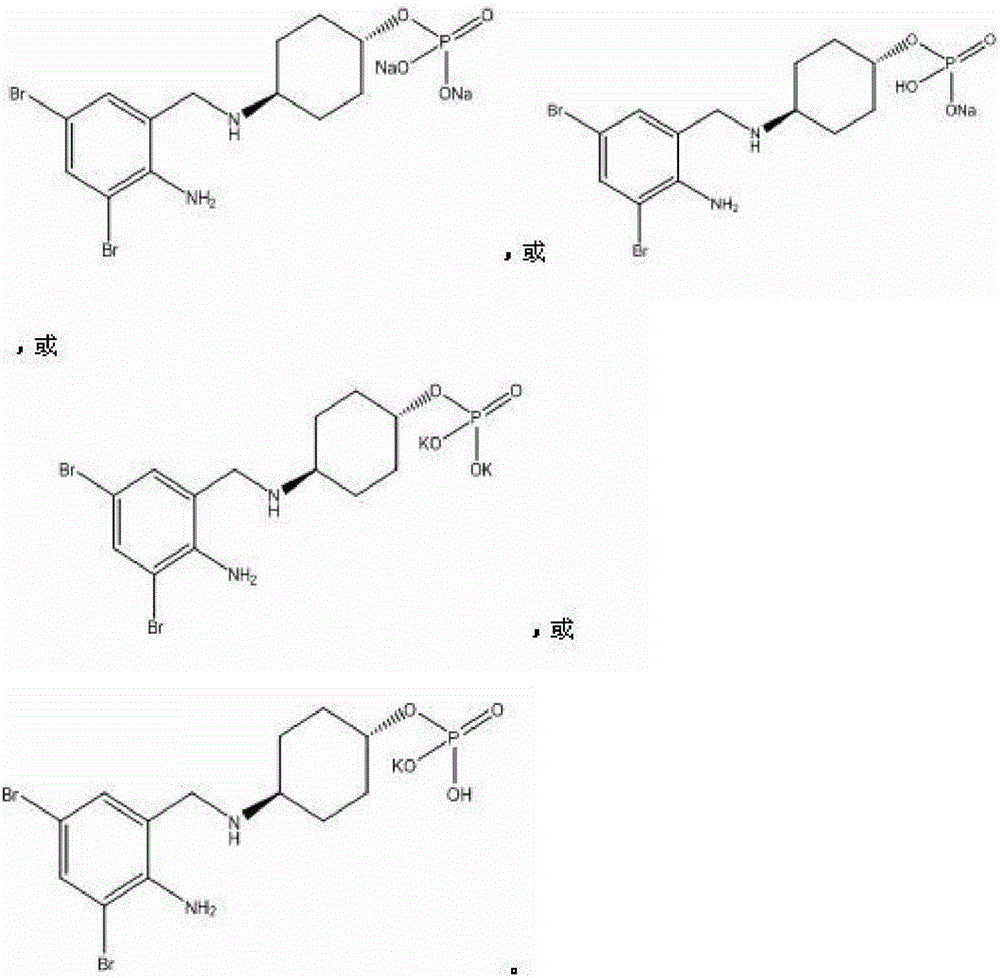
[0041] The starting material a (105mg, 0.28mmol) was dissolved in pyridine (5ml), and phosphorus oxychloride (46.5mg, 0.31mmol) was added dropwise, and reacted overnight at room temperature. Thin-layer chromatography tracked the reaction process. After the reaction was complete, it was quenched with ice water, dropped into dilute sodium hydroxide solution (0.56mmol), concentrated under reduced pressure, added acetone to precipitate a solid, and obtained compound 1 (105mg, off-white solid). The yield : 80.1%.
[0042] MSm / z(ES):502.9[M+1]
[0043] 1 HNMR (300MHz, D2O): δ (ppm) 7.33 (1H, m), 6.99 (1H, m), 3.60 (3H, brs), 2.50 (1H, brs), 1.77 (4H, brs), 1.02 (4H, brs);
[0044] Elemental Analysis: C, 31.1; H, 3.4; Br, 31.8; N, 5.6; Na, 9.1;
Embodiment 2
[0045] The preparation of embodiment 2 compound 2
[0046]
[0047] The preparation scheme is shown in the following reaction formula:
[0048] The starting material a (105mg, 0.28mmol) was dissolved in pyridine (5ml), and phosphorus oxychloride (46.5mg, 0.31mmol) was added dropwise, and reacted overnight at room temperature. Thin-layer chromatography tracked the reaction process. After the reaction was complete, it was quenched with ice water, dropped into dilute sodium hydroxide solution (0.28mmol), concentrated under reduced pressure, added acetone to precipitate a solid, and obtained compound 2 (100mg, off-white solid). The yield : 74.1%.
[0049] MSm / z(ES):480.9[M+1]
[0050] 1 HNMR (300MHz, D2O): δ (ppm) 7.34 (1H, m), 6.95 (1H, m), 3.54 (3H, brs), 2.30 (1H, brs), 1.87 (4H, brs), 1.12 (4H, brs),4.10-4.20(2H,m);
[0051] Elemental Analysis: C, 32.53; H, 3.78; Br, 33.29; N, 5.84; Na, 4.79;
Embodiment 3
[0052] The preparation of embodiment 3 compound 3
[0053]
[0054] The preparation method is shown in the following reaction formula:
[0055]
[0056] The starting material a (105mg, 0.28mmol) was dissolved in pyridine (5ml), and phosphorus oxychloride (46.5mg, 0.31mmol) was added dropwise, and reacted overnight at room temperature. Thin-layer chromatography tracked the reaction process. After the reaction was complete, it was quenched with ice water, dropped into dilute potassium hydroxide solution (0.56mmol), concentrated under reduced pressure, added acetone to precipitate a solid, and obtained compound 3 (105mg, off-white solid). The yield : 76.8%.
[0057] MSm / z(ES):534.8[M+1]
[0058] 1 HNMR (300MHz, D2O): δ (ppm) 7.31 (1H, m), 6.93 (1H, m), 3.65 (3H, brs), 2.53 (1H, brs), 1.79 (4H, brs), 1.22 (4H, brs);
[0059] Elemental Analysis: C, 29.23; H, 3.21; Br, 29.91; K, 14.64; N, 5.24;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com