Nuclear fuel pellet having enhanced thermal conductivity, and preparation method thereof
An enhanced thermal conductivity technology, which is applied in the direction of reactor fuel elements, reactor fuel materials, nuclear engineering, etc., can solve the problems that the composition cannot be prepared, and achieve the effect of enhanced plasticity, low preparation cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation method of thermal conductivity-enhanced nuclear fuel pellets in this embodiment is as follows:
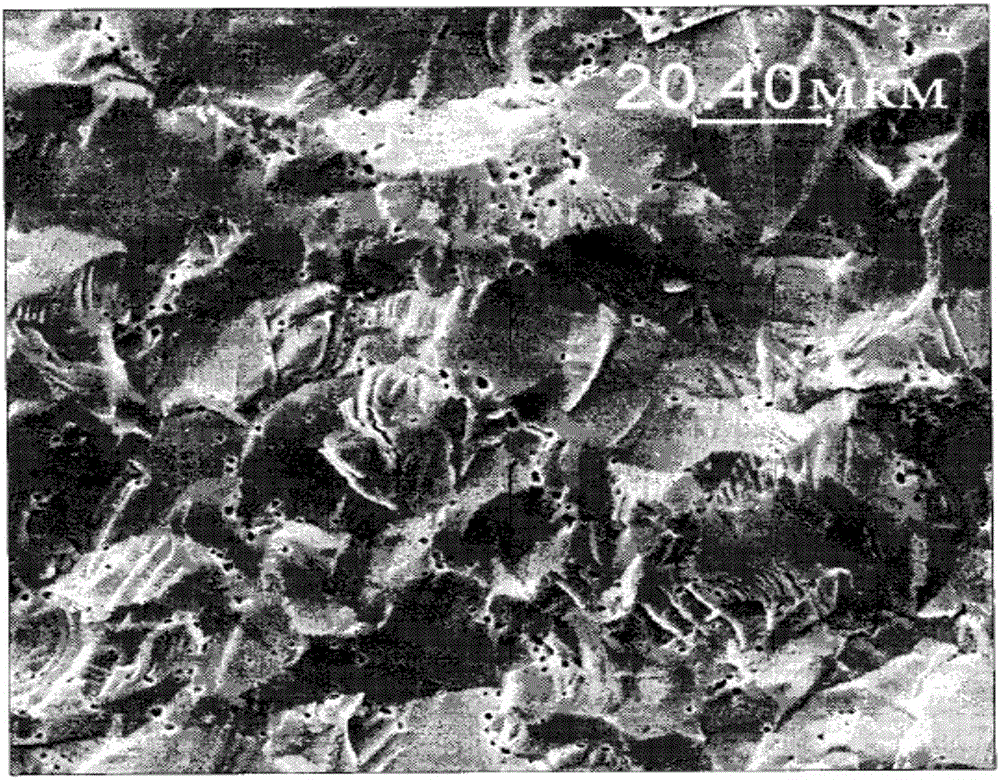
[0052] When uranyl nitrate solution and ammonia solution are added to the buffer solution at the same time, the deposition process begins at 55-60±2°C in the following two pH stages. In the first stage, the pH is maintained at 6.5-6.7. In the second stage, The pH is between 9.0 and 10.5, forming the final precipitate polyammonium polyuranate (PUA); calcination is carried out at 600 to 680°C until UO 2 Reduction; melting of metal uranium at a temperature higher than 1150°C; sintering at 1750°C in a trace amount of hydrogen-nitrogen liquid phase medium until the formation of metal clusters. Sintering in a liquid medium results in the desired porosity and pellet structure. This kind of UO can be identified by X-ray photon spectrometer 2 The new structure of the core block and the additional U-U chemical bond structure: the core block structure has small pores ...
Embodiment 2
[0054] The preparation method of thermal conductivity-enhanced nuclear fuel pellets in this embodiment is as follows:
[0055] When uranyl nitrate solution, metal additives and ammonia solution are added to the buffer at the same time, the deposition process begins at 55-60±2°C in the following two pH stages. In the first stage, the pH is maintained at 7.0-7.2, and in the In the second stage, the pH is between 8.0 and 8.5, and the final precipitate is ammonium polyuranate (PUA). The metal additive is selected from chromium, and the chromium acts as a catalyst, which can catalyze the uranium dioxide nanometer near it during the pellet sintering process. The particles are partially reduced to produce metal uranium; metal uranium is melted at a temperature higher than 1150 °C; sintering is carried out at 1750 °C in a trace amount of hydrogen-nitrogen liquid phase medium until metal clusters are formed. Sintering in a liquid medium results in the desired porosity and pellet struct...
Embodiment 3
[0057] The nuclear fuel pellets of this embodiment are prepared according to the following conventional methods:
[0058] Under the action of mechanical stirring, add 0.5wt% 4-amino-1,2,4-triazole (abbreviated as triazole) to the uranium dioxide powder prepared by conventional method; Press sintering of pellets; during sintering, ammonium-containing triazole radical ions decompose to release hydrogen, which causes a reduction reaction of uranium dioxide in its surrounding area, resulting in the formation of metal clusters and substoichiometric combinations inside the pellets metal uranium melts at a temperature higher than 1150°C; sintering is carried out at 1750°C in a trace amount of hydrogen-nitrogen liquid phase medium until metal clusters are formed, and the result of sintering in liquid phase medium is to form the required Porosity and Pellet Structure. This kind of UO can be identified by X-ray photon spectrometer 2 The new structure of the core block and the addition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com