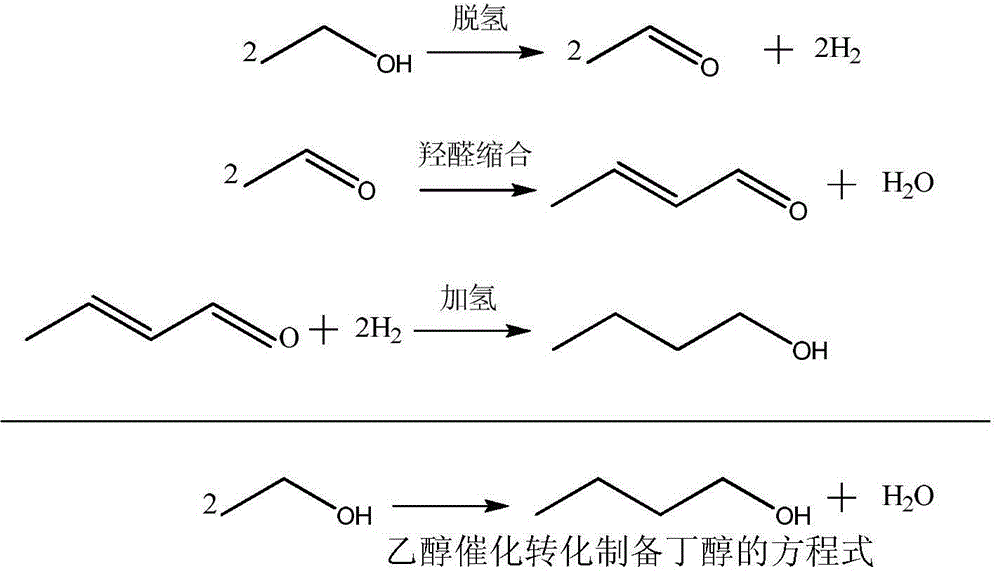
Method for producing high-carbon primary alcohols by catalytic conversion of ethanol
A technology of catalytic conversion and carbon primary alcohol, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of reduced product selectivity, low ethanol conversion rate, easy metal agglomeration, etc., and achieves good economical efficiency. The effect of stability and practicability, good stability, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Catalyst preparation
[0025] Weigh 0.04mol nitrate M (M is one or more of Ni and Co, Cu, Zn) and 0.08mol magnesium nitrate (Mg(NO 3 ) 2 ·6H 2 O), 0.02mol aluminum nitrate (Al(NO 3 ) 3 9H 2 O) was dissolved in 100mL deionized water to make solution A. Another weighed 0.12mol anhydrous sodium carbonate (Na 2 CO 3 ) was dissolved in 60mL of deionized water and mixed with 20mL of 3M NaOH solution to obtain solution B. Solution B was placed in a 35°C water bath, and solution A was added to it at a rate of 3 mL / min under vigorous stirring, and a small amount of NaOH solution was added to adjust the pH to 10. The precipitate was crystallized in a 65°C water bath for 18h. After filtering and washing, the samples were dried at 80°C.
Embodiment 2
[0030] The catalytic conversion experiment was carried out in a fixed-bed reactor, and the specific conditions were as follows: 2 g of the catalyst precursor prepared in Example 1 was added to the fixed-bed reactor, and hydrogen was reduced online at a hydrogen flow rate of 60 ml / min and a reduction temperature of 600 ° C. , restore event 2h.
[0031] After reduction, the temperature was lowered, and nitrogen gas was introduced at a pressure of 3 MPa and a gas flow rate of 20 ml / min. Rise to the reaction temperature and pump the raw materials into the reaction, and the liquid phase product and gas phase product are analyzed by gas chromatography respectively.
Embodiment 3
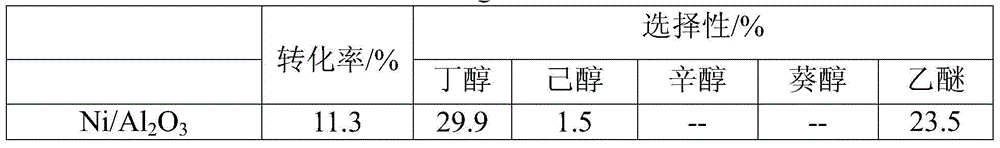
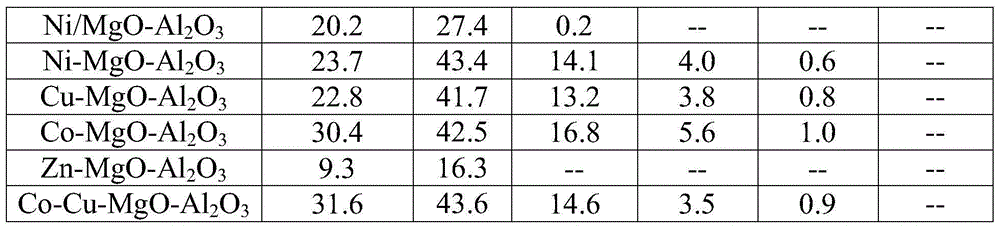
[0033] The different catalysts prepared by comparative example 1 and embodiment 1, the result of preparing even-carbon alcohols by the catalytic conversion of ethanol under the conditions of embodiment 2:
[0034] Table 1 The reaction results of ethanol catalytic conversion under different catalysts to prepare even carbon alcohols (ethanol concentration 98wt%, feed rate 0.1ml / min, atmosphere is argon, reaction temperature 250 ℃, catalyst quality 2.2g)
[0035]
[0036]
[0037] It can be seen from the reaction data that compared with the catalyst prepared by the impregnation method, the Ni, Cu, and Co catalysts prepared by the co-precipitation method showed a high conversion rate and selectivity in the conversion of ethanol, and the yield of even-carbon alcohol exceeded 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com