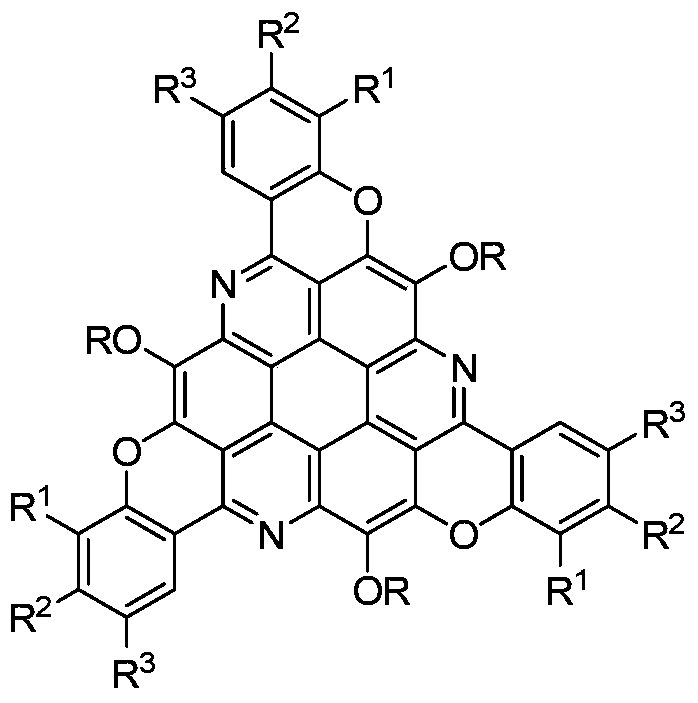
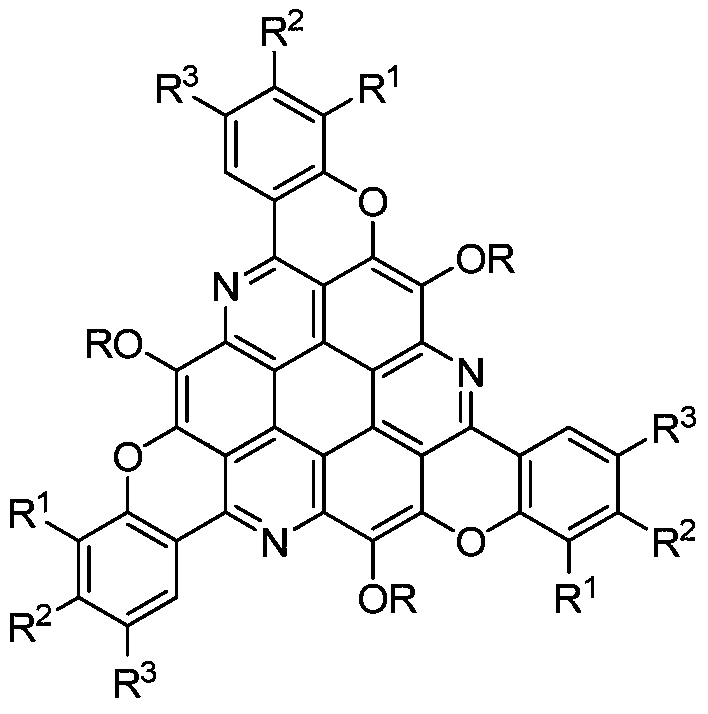
π-extended s-triazepine derivatives and their synthetic methods
A technology of mesitrazine and synthesis method, applied in the field of synthesis of nano-graphene compounds, can solve unfavorable electron transport performance and improvement of device performance, limit conjugation, hinder π-π stacking effect and self-assembly Properties and other issues, to achieve the effect of eliminating adverse effects, improving material properties and device performance, and expanding π-conjugated systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Taking the synthesis of π-extended s-triazepine derivatives with the following structural formula as an example, the specific synthesis method is as follows:
[0020]
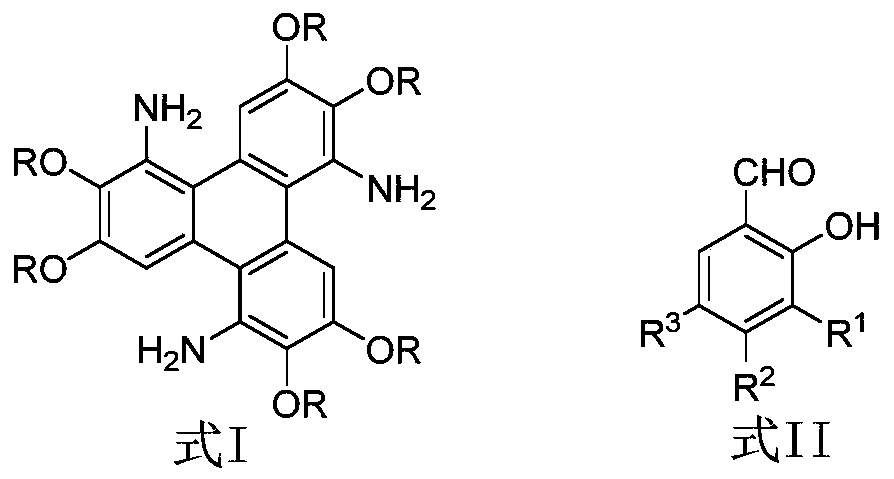
[0021] Add 0.14g (0.2mmol) 1,5,9-triamino-2,3,6,7,10,11-hexabutoxytriphenylene and 0.15g (1.2mmol) salicylaldehyde to 5mL N-formaldehyde In pyrrolidone (NMP), stir evenly at room temperature, then dropwise add 0.0014g trifluoromethanesulfonic acid, raise the temperature to 120°C, track and detect with TLC, the reaction ends after 12 hours, cool to room temperature, add 0.16g (1.2mmol) Water K 2 CO 3 , heated to 120°C with stirring, cooled to room temperature after 12 hours of reaction, diluted with 20 mL of distilled water, extracted with chloroform (20 mL x 3 times), washed repeatedly with water and saturated saline, and finally the combined organic phase with anhydrous Na 2 SO 4 Dry overnight, concentrate by rotary evaporation, and carry out column chromatography with chloroform, and the obtaine...
Embodiment 2
[0025] Taking the synthesis of π-extended s-triazepine derivatives with the following structural formula as an example, the specific synthesis method is as follows:
[0026]
[0027] The salicylaldehyde used in Example 1 is replaced with equimolar 5-tert-butyl salicylaldehyde, and other steps are the same as in Example 1 to obtain an orange-red solid, i.e., π-extended s-triazepine derivatives, and the yield It is 96%, and the melting point is greater than 300°C.
[0028] The resulting product was characterized with an Avance type superconducting Fourier digital nuclear magnetic resonance spectrometer (Switzerland, Bruker company) and a MALDI-TOF mass spectrometer (Germany, BurkerDaltonics), and the characterization data were as follows:
[0029] 1 H NMR (400MHz, CDCl 3 +TMS)δ(ppm): 8.93(s, 3H), 7.56(dd, J=8.2, 2.4Hz, 3H), 7.13(d, J=8.6Hz, 3H), 4.84(t, J=6.1Hz, 6H), 2.20-2.13(m, 6H), 2.03-1.94(m, 6H), 1.63(s, 27H), 1.20(t, J=7.3Hz, 9H); 13 C NMR (75MHz, CDCl 3 +TMS)δ(ppm)...
Embodiment 3
[0031] Taking the synthesis of π-extended s-triazepine derivatives with the following structural formula as an example, the specific synthesis method is as follows:
[0032]
[0033] The salicylaldehyde used in Example 1 is replaced with equimolar 4-methoxy salicylaldehyde, and other steps are the same as in Example 1 to obtain an orange-red solid, that is, π-extended s-triazepine derivatives, and the yield It is 85%, and the melting point is greater than 300°C.
[0034] The resulting product was characterized with an Avance type superconducting Fourier digital nuclear magnetic resonance spectrometer (Switzerland, Bruker company) and a MALDI-TOF mass spectrometer (Germany, BurkerDaltonics), and the characterization data were as follows:
[0035] 1 H NMR (300MHz, CDCl 3 +TMS) δ (ppm): 8.17 (d, J = 8.6Hz, 3H), 6.68 (d, J = 6.9Hz, 3H), 6.43 (s, 3H), 4.53 (t, J = 6.0Hz, 6H) , 3.87(s, 9H), 1.96-1.89(m, 6H), 1.81-1.74(m, 6H), 1.15(t, J=7.2Hz, 9H); 13 C NMR (75MHz, CDCl 3 +TM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com