Novel solid-state phase transfer catalyst based on Cd-MOF, method for preparing novel solid-state phase transfer catalyst and application thereof
A cd-mof-1, C27H19N4O2 technology, applied in the field of catalyst preparation, can solve the problems of complicated operation, difficult catalyst recovery and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation of organic ligand L
[0075] Concrete preparation steps are as follows:
[0076] (1) 4,4-dibromobenzil (10.00g, 27.17mmol), ammonium acetate (41.89g, 543.45mmol), paraformaldehyde (1.63g, 54.34mmol), join in 100mL glacial acetic acid solution, stir Heat to reflux for 5 hours, adjust to neutral with saturated sodium carbonate solution after the reaction, extract with 200mL ethyl acetate, wash with sodium carbonate solution, extract the aqueous phase with ethyl acetate, combine the organic phases, and wash with anhydrous magnesium sulfate Dry, filter, and remove the solvent under reduced pressure to obtain 10.07 g of a yellow solid with a yield of 98.00%.
[0077]
[0078] (2)N 2 Under protection, intermediate A (10.58mmol, 4.00g) and sodium hydride (11.64mmol, 0.28g) were placed in a 100ml three-necked flask, and 50ml of anhydrous THF was slowly added as a solvent, heated to 80°C, and stirred at a constant temperature of 80°C for 1h , ...
Embodiment 2
[0083] Embodiment 2: the synthesis of Cd-MOF-1
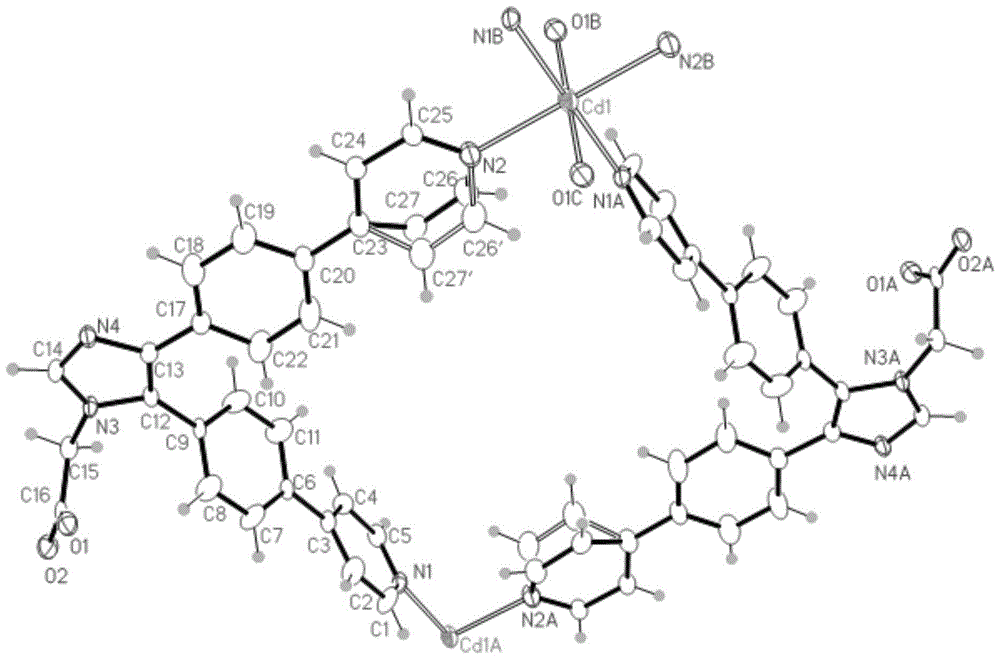
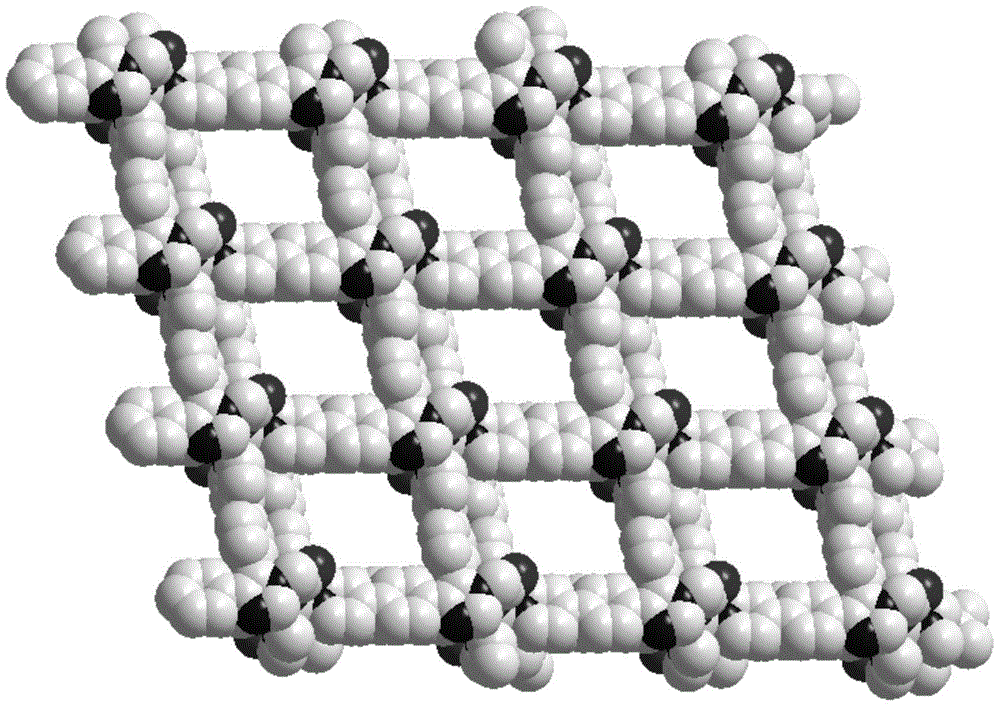
[0084] The organic ligand L (8.60mg, 0.02mmol) prepared in Example 1 and cadmium acetate (0.01mmol, 2.13mg) were dissolved in 2mL of methanol, placed in a 5ml small test tube, and kept at 120°C for 72 hours. After 50 hours, the temperature was cooled to room temperature to obtain colorless blocky crystals [Cd(C 27 h 19 N 4 o 2 ) 2 ] n , n is a non-zero natural number, the yield is 4.3 mg, and the yield is 44% (based on L).
[0085] We characterized the compound by IR and TGA, and the results are shown in 10 and 11, respectively.
Embodiment 3
[0086] Example 3: Synthesis of Cd-MOF-1'
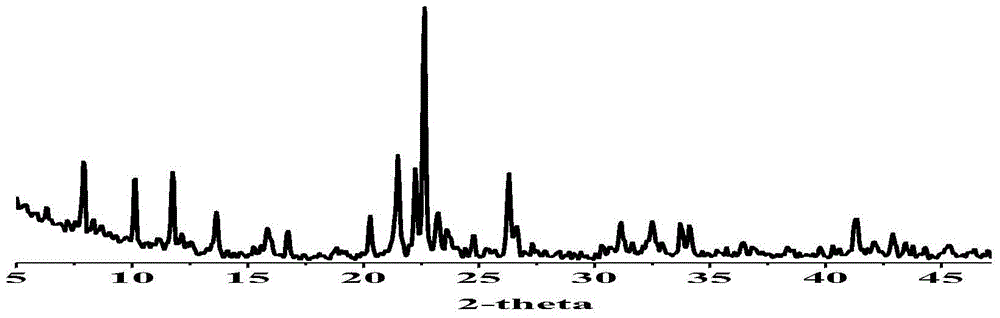
[0087] Put the Cd-MOF-1,1-bromopropane prepared in Example 2 into a 25ml two-necked bottle, add 10ml of anhydrous acetonitrile as a solvent, heat to 80°C-90°C, stir for 48 hours, stop heating, and let the reaction system decompose. to room temperature, centrifuged, collected the precipitate, washed twice with acetonitrile, washed twice with methanol, and washed twice with ether to obtain a three-phase catalyst metal-organic framework Cd-MOF-1′, which we characterized by IR and TGA , see the results respectively Figure 12 , 13 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com