A reagent card for detecting the curative effect of thienopyridine antiplatelet drugs and its use method and application
A technology of anti-platelet drugs and detection reagents, which is applied in the direction of testing pharmaceutical preparations, biological tests, material inspection products, etc., to achieve practical effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
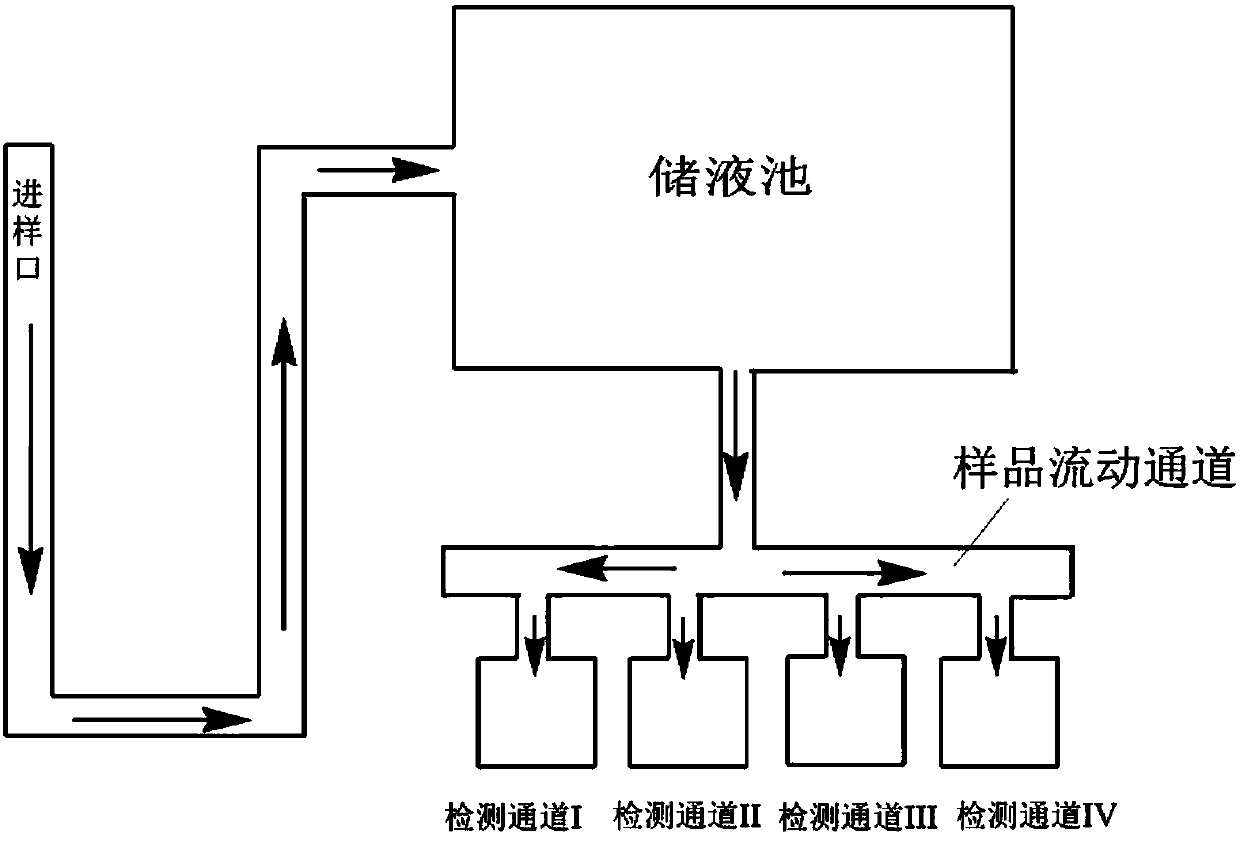
Image
Examples
Embodiment 1
[0060] microsphere labeling
[0061] (1) Washing balls: take 100 μL of fluorescent microspheres, wash twice with 1 mL of MES pH4.5 buffer, centrifuge at 8000 g, 4°C for 10 min, discard the supernatant, and reconstitute to 1 mL by adding MES pH4.5 after ultrasonication for one minute.
[0062] (2) Activation of microspheres: Add EDC and NHS solutions to a final concentration of 0.5mg / mL, activate at room temperature for 15 minutes, centrifuge at 4°C (8000g, 10min), discard the supernatant, and use 0.02M pH8.5 borate buffer 1 mL was washed twice and reconstituted to 1 mL with this buffer.
[0063] (3) Add labeled protein: Sonicate the activated microspheres for one minute, mix with a vortex mixer, add GpIIb / IIIa receptor ligand to a final concentration of 0.5 mg / mL, and incubate at room temperature for 2 hours.
[0064] (4) Blocking: after the incubation, BSA with a final concentration of 0.5% was added and blocked at room temperature for 30 min.
[0065] (5) Centrifugation: A...
Embodiment 2
[0073] 1. Inhibition of GpIIb / IIIa receptor activity
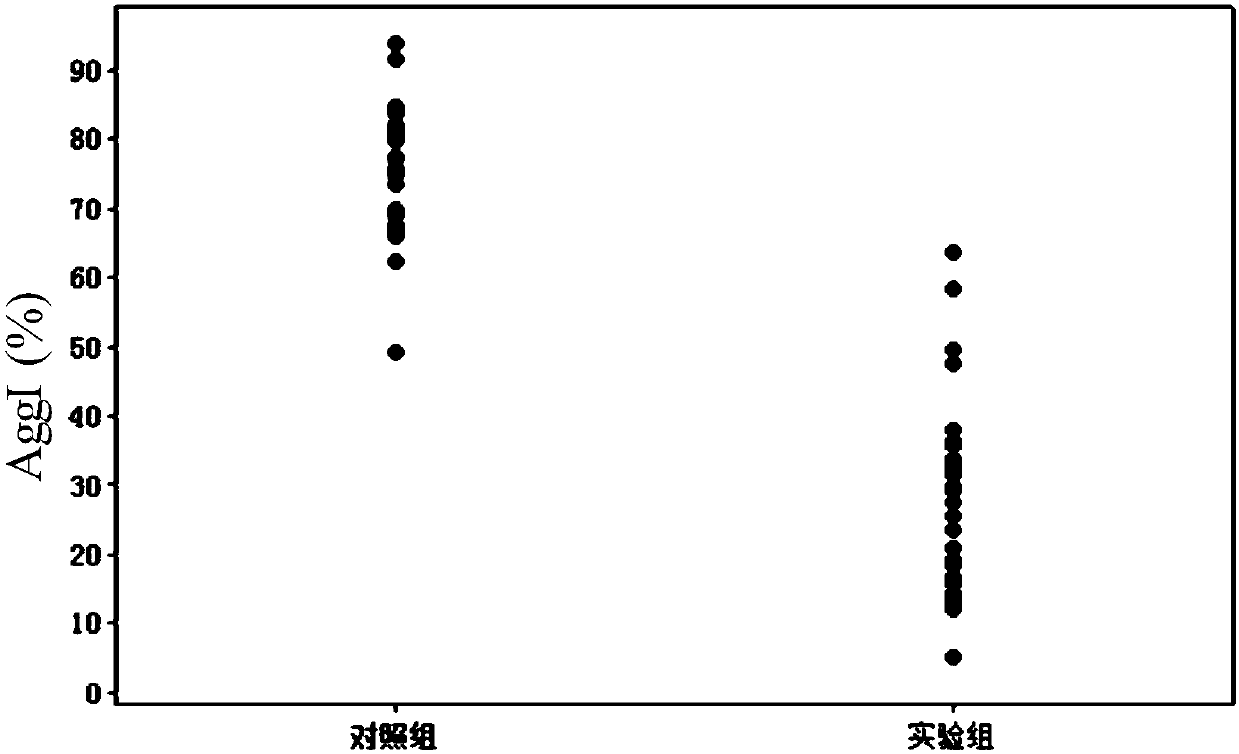
[0074] Blood samples were collected from 31 healthy volunteers, anticoagulated with 3.2% sodium citrate, and 2 tubes were taken from each case, each 2 mL. An appropriate amount of GpIIb / IIIa receptor activity inhibitor abciximab (Reopro) was dissolved in 0.01M PBS buffer to prepare 0.15mM. One blood sample was taken from each case as the experimental group, 2 μL of 0.15 mM abciximab was added respectively, and the final concentration of abciximab in the blood sample was 0.15 μM. Gently invert the blood sample 10 times to mix the abciximab and the blood sample thoroughly, and incubate at 37 degrees for 1 hour. 2 μL of 0.01M PBS buffer solution was added to each remaining blood sample, and incubated at 37°C for 1 hour, which was used as a control group.
[0075] GpIIb / IIIa receptor activity assay
[0076] After the above-mentioned experimental incubation, take the blood samples of the experimental group and detect the GpI...
Embodiment 3
[0078] Clopidogrel Drug Inhibition Rate Evaluation Test
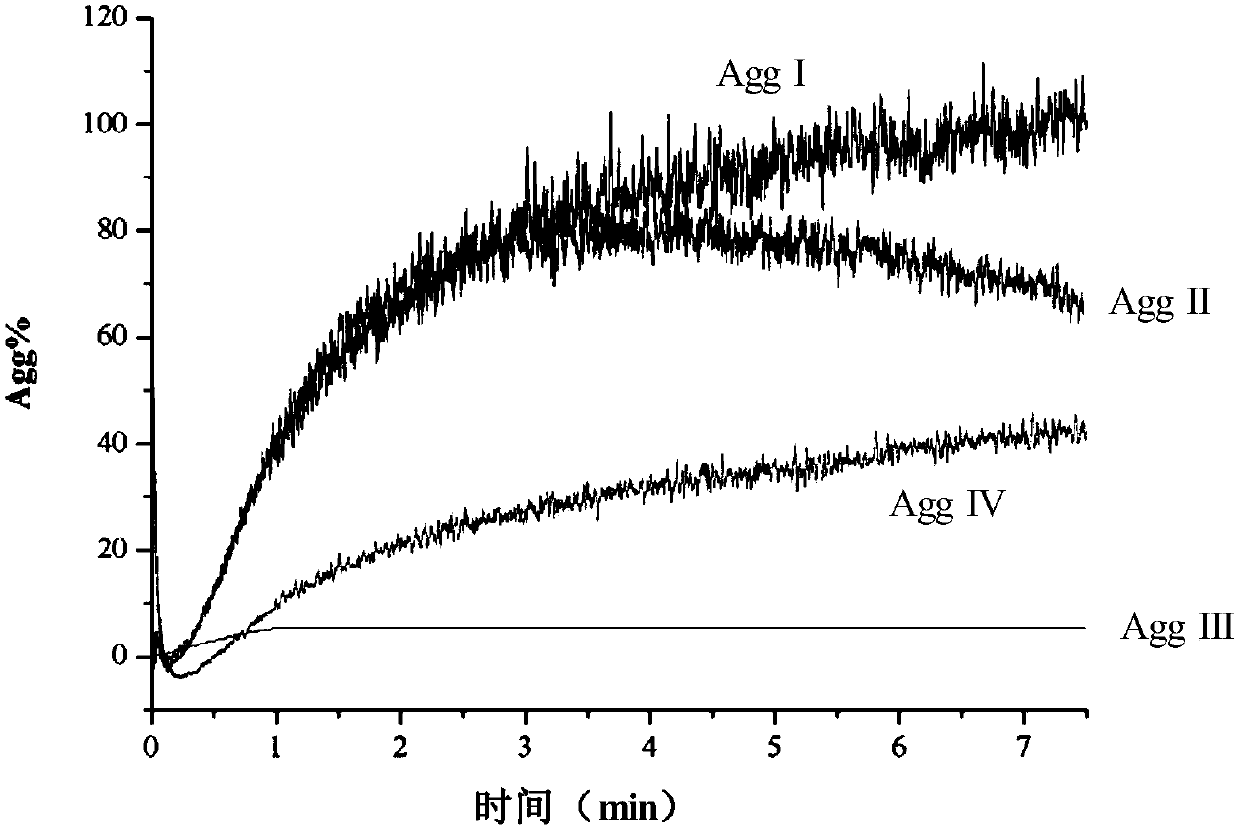
[0079]Collect 2 mL of sodium citrate whole blood from patients taking clopidogrel, and gently invert the blood collection tube several times to mix well. Insert the thienopyridine antiplatelet drug curative effect detection reagent card into the matching optical turbidimetric detection equipment, insert the blood collection tube into the sampling needle of the reagent card, and click Start to start the detection. The instrument automatically detects the change degree of the light transmittance of the four detection slots of the reagent card, and converts it into an electrical signal through the corresponding software, draws the change curve of the electrical signal, and finally gives the platelet aggregation rate and drug inhibition rate. For platelet aggregation detection results of the four channels of the reagent card, see image 3 . The platelet aggregation percentage PAP% of the ADP activation pathway measured in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com