Polyacid @aluminum oxide composite catalytic material and preparing method thereof
A technology of composite catalytic materials and alumina, which is applied in the application field of catalytic thioether oxidation reaction, can solve problems such as not easy to be affected by active components, loss of multi-acid, increase of active components, etc., to achieve the reaction time and the amount of hydrogen peroxide The effect of reducing, good catalytic performance and improving cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
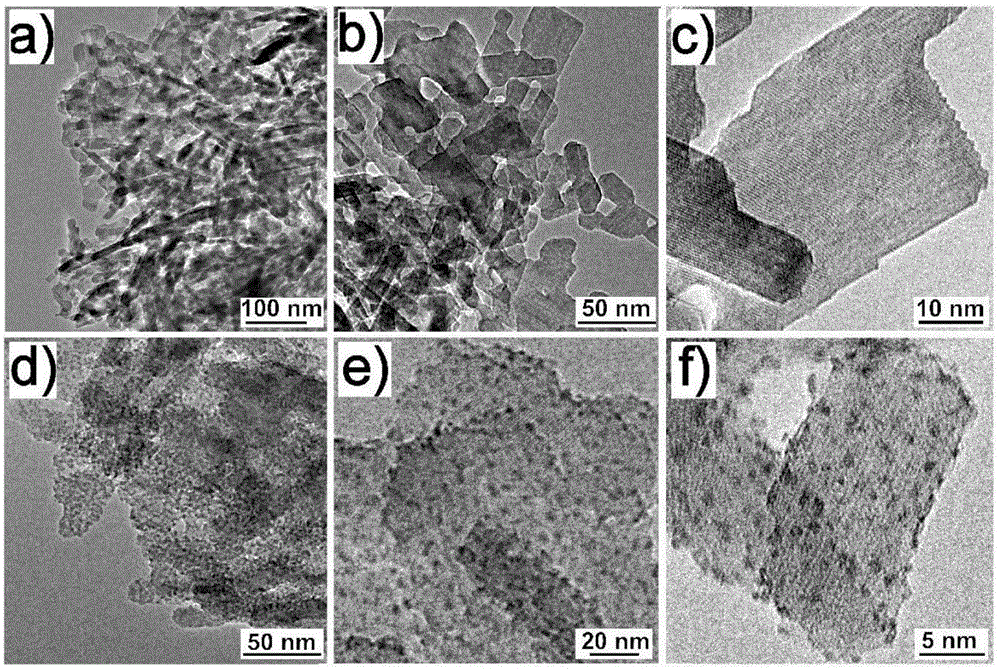
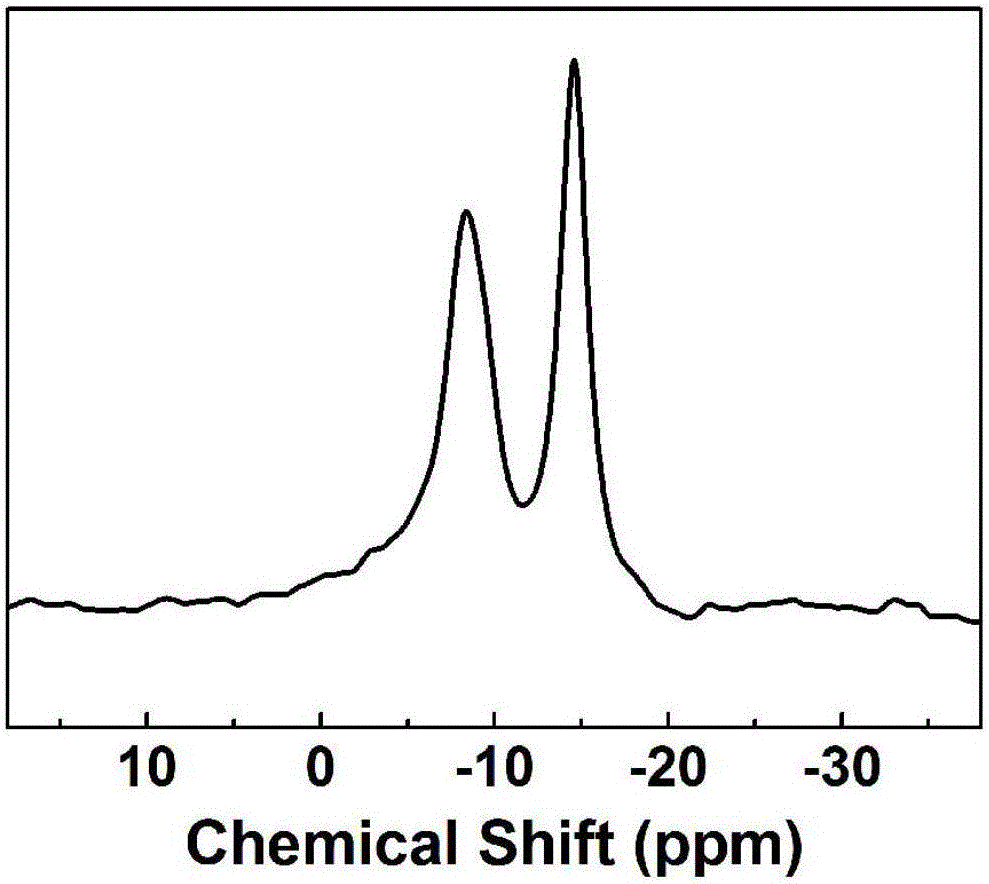
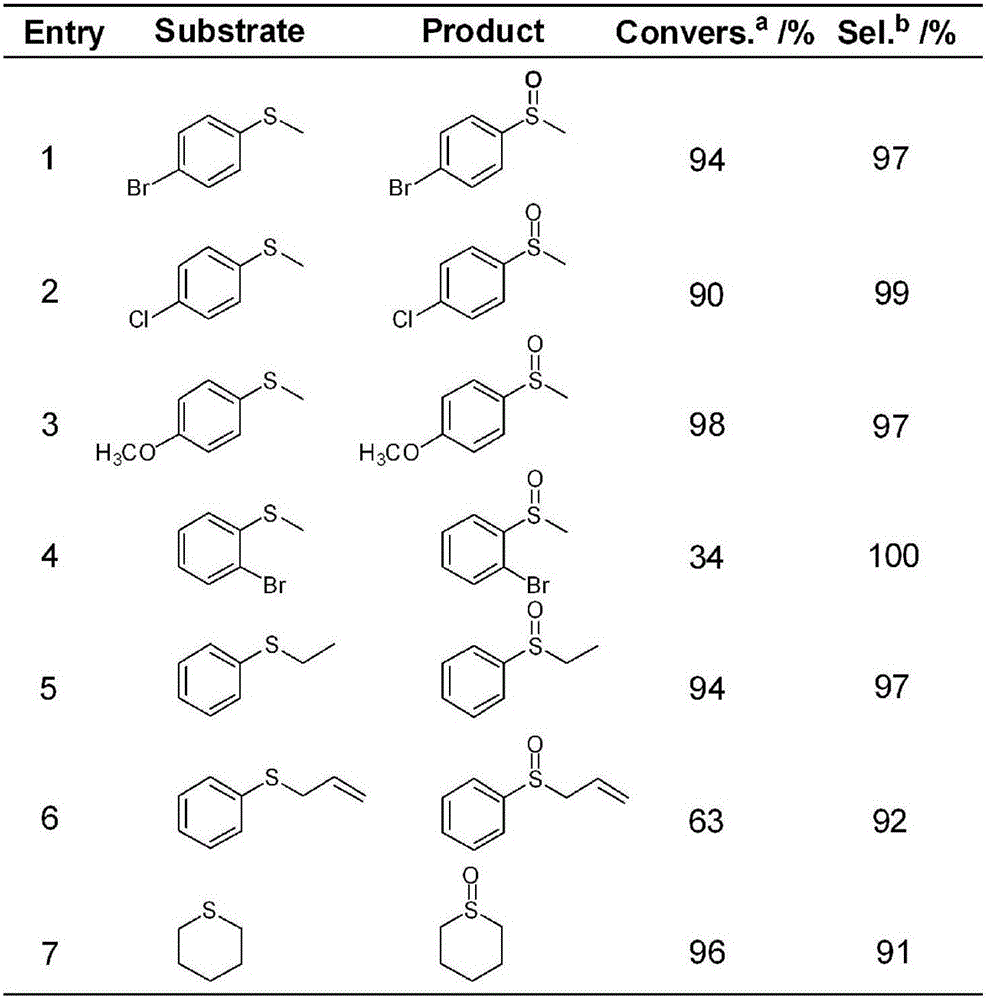
Image
Examples
Embodiment 1
[0020] 1) Calcining γ-alumina balls at 500°C for 22 hours and cooling to room temperature;
[0021] 2) Add 4g of Na 12 [α-P 2 W 15 o 56 ]·24H 2 O and 20g of calcined γ-alumina balls were dispersed in 500mL of water, the pH value of the solution was adjusted to 1.5 with hydrochloric acid, the magnetic stirring reaction was performed at room temperature for 2 hours, the temperature was raised to 80°C and the magnetic stirring reaction was continued for 2 hours, filtered, and 2mol / L lithium chloride solution to wash the product three times, and vacuum-dry at 60° C. for 12 hours to obtain a multi-acid alumina nanocomposite catalytic material.
[0022] The multi-acid alumina nanocomposite catalytic material prepared above is used to catalyze the oxidation reaction of thioether: weigh 2.5 μmol of the synthesized polyacid alumina nanocomposite catalytic material in a round bottom flask, then successively add 1mmol sulfide anisole, Add 1mmol hydrogen peroxide and 200μL methanol ...
Embodiment 2
[0026] 1) Calcining gamma-alumina balls at 500°C for 25 hours and cooling to room temperature;
[0027] 2) Add 4g of Na 8 H[A-PW 9 o 34 ]·7H 2 O and 20 g of calcined γ-alumina balls were dispersed in 500 mL of water, and the pH value of the solution was adjusted to 0.5 with hydrochloric acid. The reaction was carried out under magnetic stirring at room temperature for 3 hours, and the temperature was raised to 85°C to continue the magnetic stirring reaction for 2 hours. The product was washed three times, and vacuum-dried at 60° C. for 12 hours to obtain a multi-acid alumina nanocomposite catalytic material.
[0028] The multi-acid alumina nano-composite catalytic material prepared above is used to catalyze the oxidation reaction of thioether: weigh 2.5 μmol of the synthesized multi-acid alumina nano-composite catalytic material in a round bottom flask, then successively add 1 mmol sulfide anisole, Add 1mmol hydrogen peroxide and 200μL methanol into a round-bottomed flask,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com