Preparation method of difluoro-sulfonyl imide lithium
A technology of lithium bisfluorosulfonyl imide and bischlorosulfonimide, which is applied in the field of preparation of lithium bisfluorosulfonyl imide, can solve the problem of low purity of lithium bisfluorosulfonyl imide, various types of raw materials and reagents, There are many types and contents of impurities, etc., to achieve good fluorination effect, good economic and environmental benefits, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
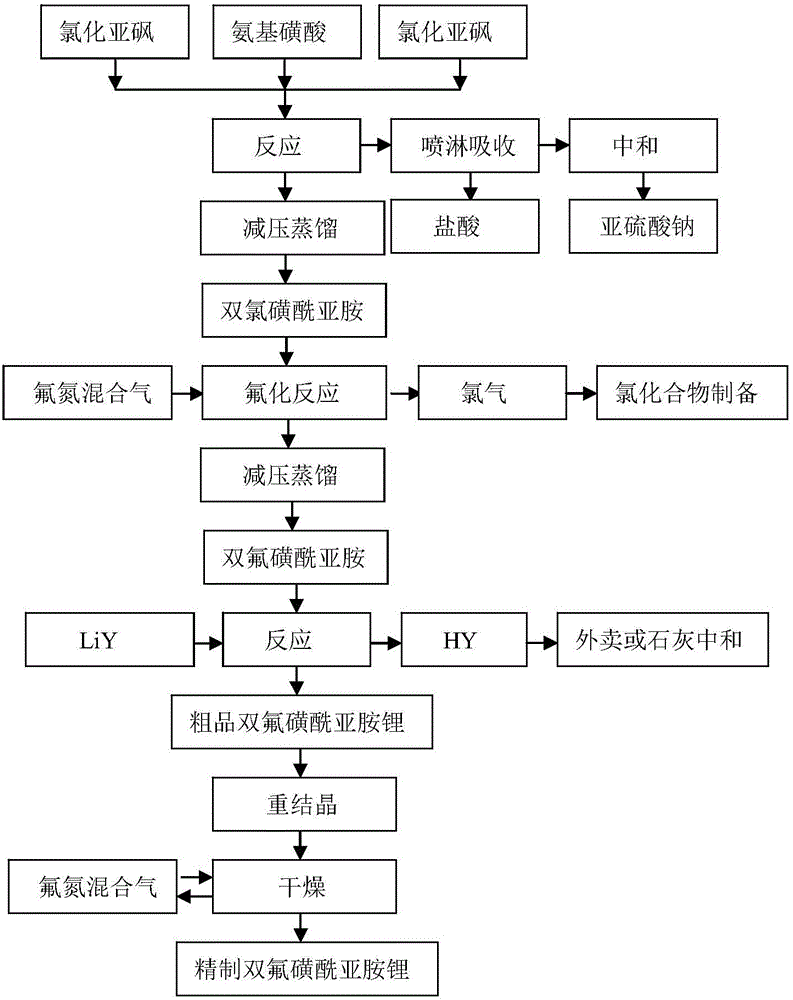
[0058] The preparation method of the bisfluorosulfonyl imide lithium of the present embodiment, such as figure 1 shown, including the following steps:
[0059] 1) Preparation of bischlorosulfonimide:
[0060] a) Under nitrogen protection, according to the ratio of 2.0:1.0:1.0 in the molar ratio of thionyl chloride, sulfamic acid and chlorosulfonic acid, 250g (100%, folded) of thionyl chloride and 102g (100%, 100% sulfamic acid was added to the reactor, and after stirring at room temperature, 123g (100%, 100%) chlorosulfonic acid was added at a constant speed, and the addition time was controlled to be 5h;
[0061] After the addition of chlorosulfonic acid, continue to stir and react at room temperature for 1 hour, then open the outlet valve of the reactor, discharge and recover the unreacted thionyl chloride and protective atmosphere in the reactor; the remaining liquid in the reactor is mixed solution A;
[0062] b) After the pressure in the reactor is stable, the liquid in...
Embodiment 2
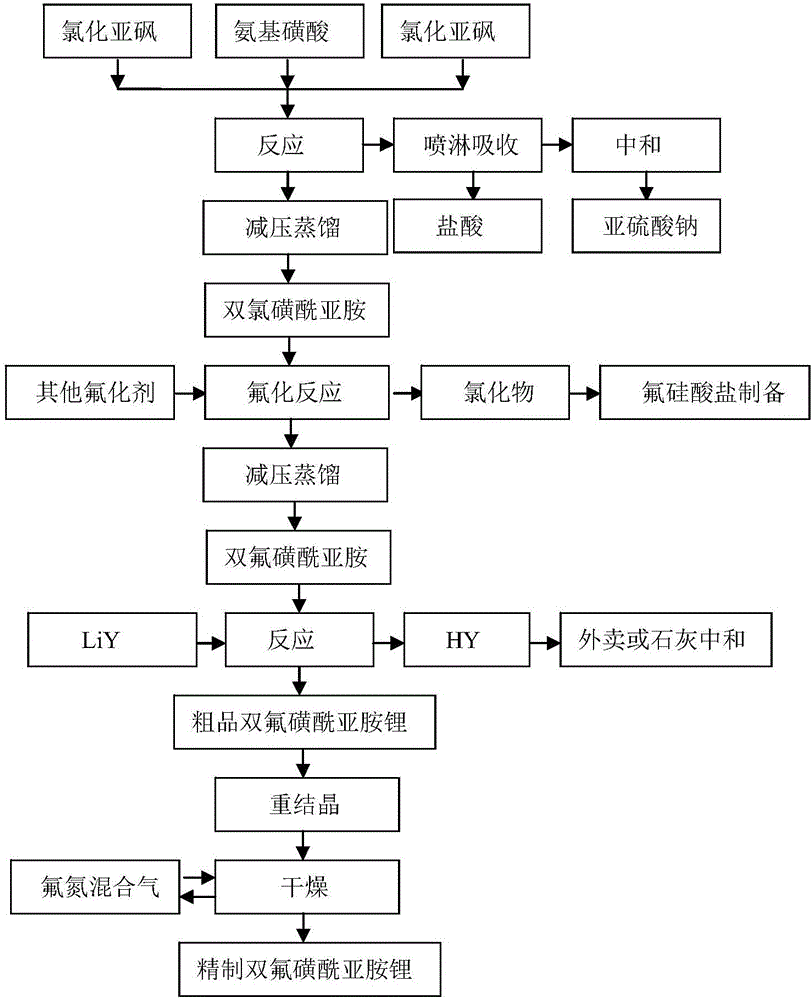
[0071] The preparation method of the lithium bisfluorosulfonyl imide of the present embodiment (the process flow diagram is the same as that of Example 1), comprises the following steps:
[0072] 1) Preparation of bischlorosulfonimide:
[0073] a) Under nitrogen protection, according to the ratio of 2.5:1.0:1.0 in the molar ratio of thionyl chloride, sulfamic acid and chlorosulfonic acid, 250g (100%, folded) thionyl chloride and 81.5g (100% , 100 percent) sulfamic acid was added to the reactor, and after stirring at room temperature, 98 g (100 percent, 100 percent) chlorosulfonic acid was added at a constant speed, and the addition time was controlled to be 5 hours;
[0074] After the addition of chlorosulfonic acid, continue to stir and react at room temperature for 0.5h, then open the outlet valve of the reactor to discharge and recover the unreacted thionyl chloride and protective atmosphere in the reactor; the remaining liquid in the reactor is mixed liquid A ;
[0075] b)...
Embodiment 6
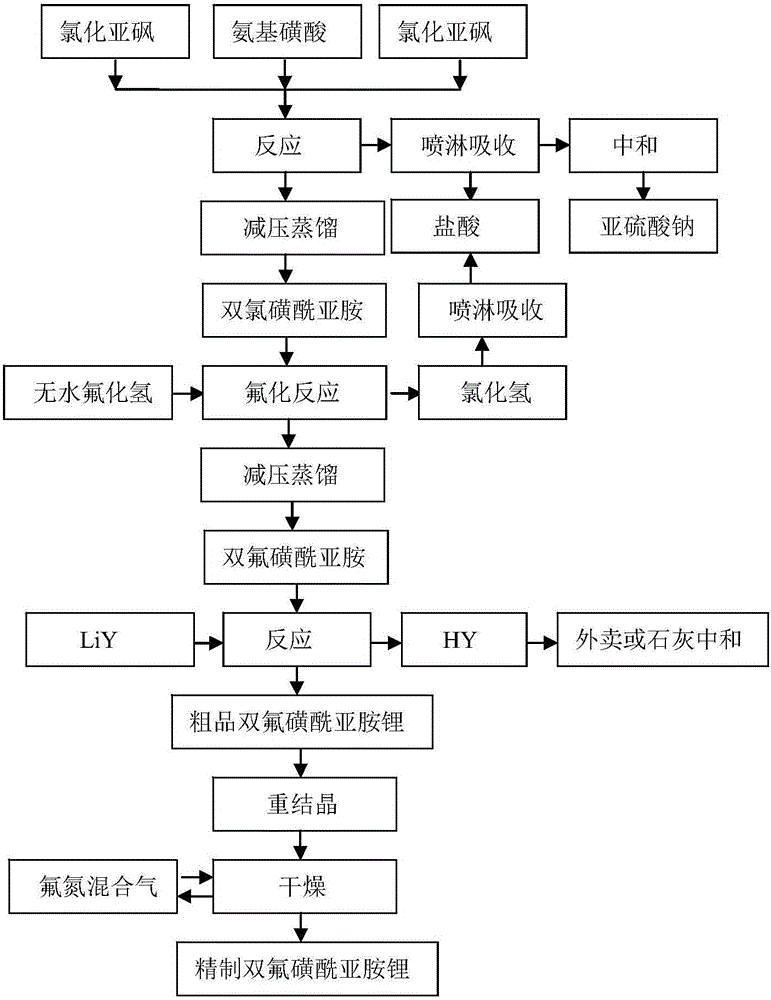
[0088] The preparation method of the bisfluorosulfonyl imide lithium of the present embodiment, such as figure 2 shown, including the following steps:
[0089] 1) Preparation of bischlorosulfonimide:
[0090] a) Under nitrogen protection, according to the ratio of 2.1:1.0:1.0 in the molar ratio of thionyl chloride, sulfamic acid and chlorosulfonic acid, 250g (100%, folded) thionyl chloride and 97.8g (100% , 100% sulfamic acid was added to the reaction kettle, and after stirring at room temperature, 117.6g (100%, 100% percent) chlorosulfonic acid was added at a constant speed, and the addition time was controlled to be 4h;
[0091] After the addition of chlorosulfonic acid, continue to stir and react at room temperature for 0.5h, then open the outlet valve of the reactor to discharge and recover the unreacted thionyl chloride and protective atmosphere in the reactor; the remaining liquid in the reactor is mixed liquid A ;
[0092] b) After the pressure in the reaction kettl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com