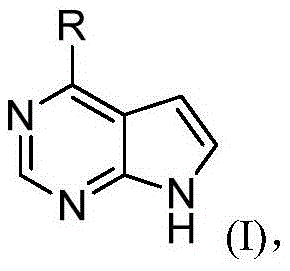
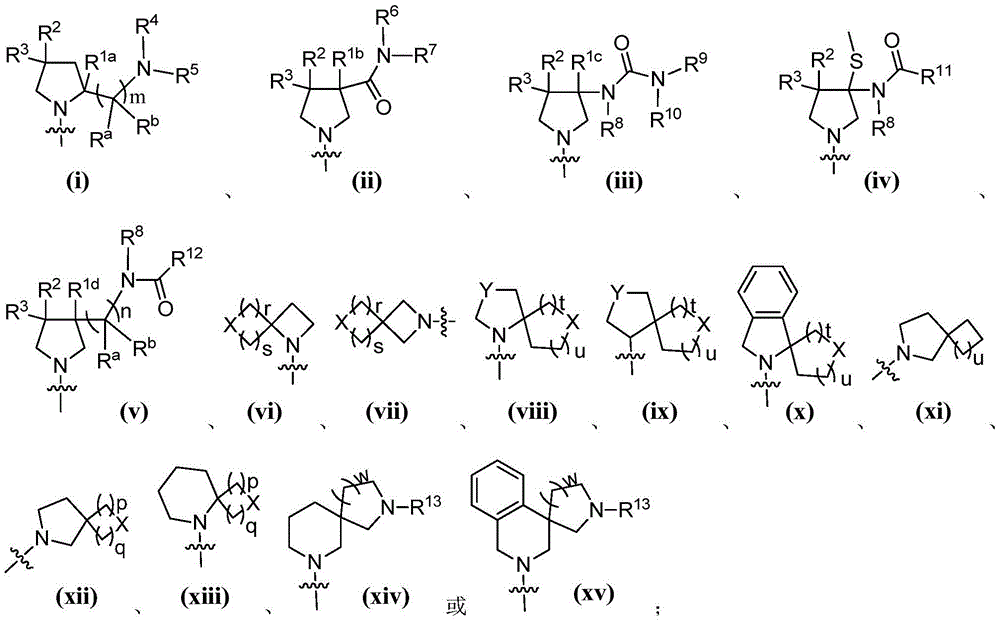
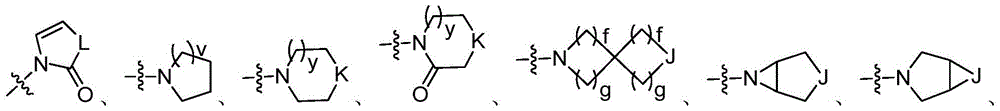Hetero-aromatic compounds and applications thereof in pharmacy
A compound, heteroaryl technology, applied in drug combinations, antipyretics, digestive system, etc., can solve problems such as signal conduction disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] Example 1 (R)-N-((1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-2-yl)methyl)-2-cyano-N-methyl Acetamide
[0331]
[0332] Step 1: Synthesis of compound (R)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid
[0333] At room temperature, D-proline (10.0g, 86.80mmol) was dissolved in tetrahydrofuran (16mL) and water (40mL), and sodium bicarbonate (18.2g, 217.14mmol) and di-tert-butyl dicarbonate (27.3 mL, 130.20mmol), react overnight at room temperature, quench with water (50mL), extract with ethyl acetate (150mL×3), and use anhydrous Na 2 SO 4 Drying, solvent removal, concentrated solution is carried out column chromatography separation (eluent: CH 2 Cl 2 / MeOH (v / v)=30 / 1), to obtain 9.3 g of colorless oil, yield: 50%.
[0334] MS(ESI,pos.ion)m / z:160.1[M-t-Bu+2] + .
[0335] Step 2: Synthesis of compound (R)-tert-butyl 2-(methylcarbamoyl)pyrrolidine-1-carboxylate
[0336] At room temperature, (R)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid (1.30g, 6...
Embodiment 2
[0349] Example 2 (S)-N-((1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-2-yl)methyl)-2-cyanoacetamide
[0350]
[0351] Step 1: Synthesis of compound (S)-tert-butyl-2-(((methylsulfonyl)oxy)methyl)pyrrolidine-1-carboxylate
[0352] At 0°C, dissolve tert-butyl L-prolinol-1-carboxylate (5.0g, 24.8mmol) in dichloromethane (50mL), add triethylamine (5.16mL, 38.2mmol), and slowly drop Add methanesulfonyl chloride (2.94mL, 38.2mmol), move to room temperature and stir overnight, the reaction solution is washed with saturated ammonium chloride solution (20mL×2), dried over anhydrous sodium sulfate, concentrated and separated by column chromatography (eluent: PE / EtOAc (v / v)=5 / 1), 6.0 g of colorless oil was obtained, yield: 87%.
[0353] MS(ESI,pos.ion)m / z:224.1[M-t-Bu+2] + .
[0354] Step 2: Synthesis of compound (S)-tert-butyl-2-(azidomethyl)pyrrolidine-1-carboxylate
[0355] At room temperature, add DMF (3 mL) to (S)-tert-butyl-2-(((methylsulfonyl)oxy)methyl)pyrrolidine-1-carbox...
Embodiment 3
[0368] Example 3 (S)-2-cyano-N-((4,4-difluoro-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-2-yl) Methyl)-N-methylacetamide
[0369]
[0370] Step 1: Synthesis of compound (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid
[0371] Dissolve (2S)-4-oxopyrrolidine-2-carboxylic acid (5.00g, 30.20mmol) in tetrahydrofuran (150mL), add triethylamine (15mL) and Boc 2 O (7 mL), stirred overnight at room temperature. After tetrahydrofuran was evaporated under reduced pressure, hydrochloric acid (1M) was added to acidify to pH 2, extracted with ethyl acetate (200mL×3), the organic phase was washed with water (150mL), saturated sodium chloride solution (150mL), and anhydrous sulfuric acid Sodium-dried, concentrated under reduced pressure and fully dried, it was directly used in the next step of synthesis.
[0372] Step 2: Synthesis of compound (S)-diethyl 1-tert-butyl-2-methyl-4-oxopyrrolidine-1,2-dicarboxylate
[0373] Dissolve (S)-1-(tert-butoxycarbonyl)-4-oxopyrroli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com