Corin gene variant and application thereof
A gene variant and mutation technology, applied in the field of clinical medicine and molecular biology, can solve the problems of unclear pathogenesis and unclear pathogenic mechanism of patients, and achieve highly specific effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Collection of blood samples.
[0027] Research objects: Patients with hypertension and pregnancy-induced hypertension were taken as the research objects. The diagnostic criteria of hypertension were based on the 1999 WHO / ISH blood pressure classification and standards. The clinical diagnostic criteria of pregnancy-induced hypertension are as follows: hypertension occurs after 20 weeks of pregnancy, and the random blood pressure is ≥140 / 90mmHg twice at intervals of 4 hours. The normal control group had no history of essential hypertension, chronic liver disease, kidney disease, diabetes, heart disease, and hyperthyroidism, and no underlying diseases such as smoking, drinking, blood transfusion, and immunotherapy.
[0028] Sample collection: venous blood collection using EDTA anticoagulant tubes, centrifugation at 3000 rpm for 10 minutes at room temperature, separation of plasma and blood cells, aliquoting and storage at -80°C for later use.
Embodiment 2
[0029] Example 2: Extraction and PCR amplification of genomic DNA.
[0030] Extraction of genomic DNA from blood cells: Use the whole blood DNA extraction kit (QIAampDNAMiniandBloodMiniKit) produced by Qiagen, USA, and follow the instructions in the kit to extract genomic DNA from the sample, and store it at -20°C.
[0031] Design of PCR primers: According to the sequence of human Corin gene in Genbank, the Primer5.0 software was used to design Corin exon amplification primers. The specific primer sequences are shown in Table 1.
[0032]
[0033]
[0034]
[0035]
[0036] PCR amplification and sequence analysis: PCR amplification conditions: pre-denaturation at 94°C for 6 min; denaturation at 94°C for 50 s, extension at 58°C for 32 s, annealing at 72°C for 32 s, and extension at 72°C for 8 min after 35 cycles. PCR products were sequenced and analyzed. The sequencing results were compared with the human Corin mRNA sequence (NM_006587.3) on the NCBI website using C...
Embodiment 3
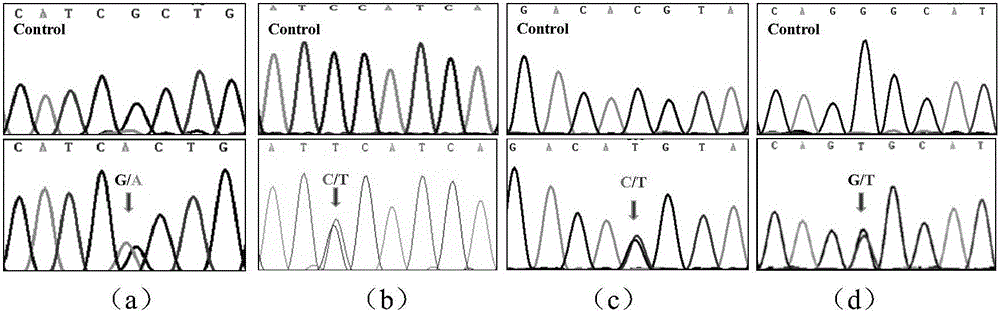
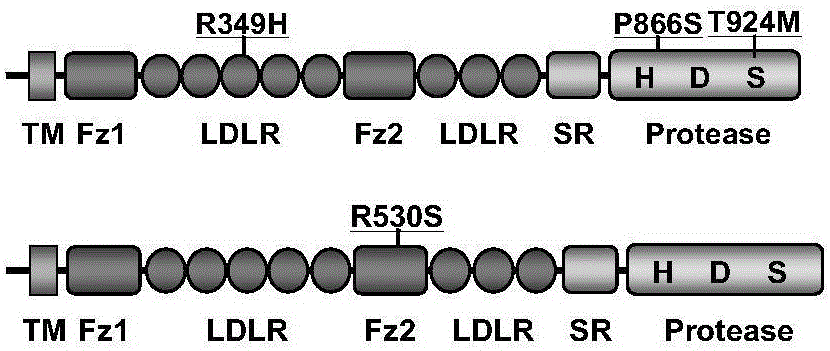
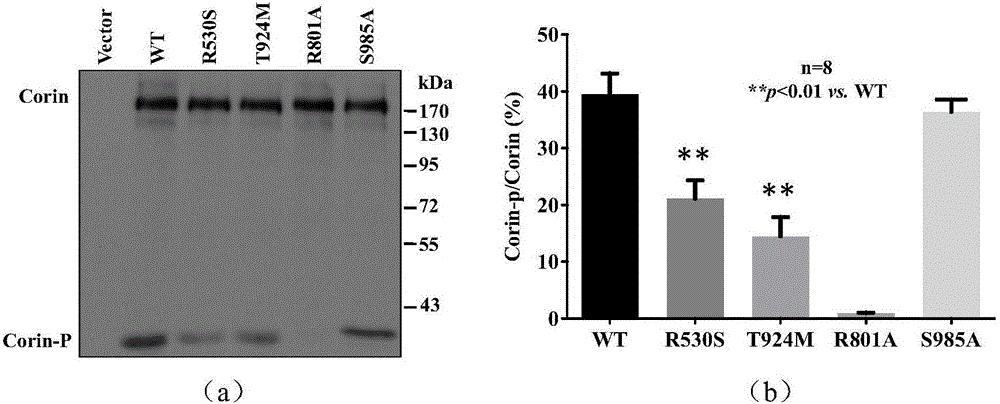
[0037] Example 3: Identification of Corin gene mutations or polymorphisms and research on their relationship with diseases.
[0038] In order to judge whether the found Corin gene variation is mutation or polymorphism, the distribution of Corin gene variation in normal population and hypertensive patients was analyzed by PCR product sequencing or quantitative PCR. Taking normal people and hypertensive patients as research objects (n>100 cases / group), peripheral blood was collected, genomic DNA was extracted, the exon where the gene was changed was amplified, and the PCR product was sequenced and analyzed. The sequencing results were compared with the human Corin mRNA sequence (NM_006587.3) in the NCBI website using Chromas2.31 software.
[0039] If the corresponding variation is not found in the normal population, it indicates that the variation is a gene mutation; if the variation frequency in the normal population is greater than or equal to 1%, it indicates that the variati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com