Application of transcription factor ZEB1 for preparing medicine capable of accelerating breast cancer chemosensitivity
A technology of transcription factor and chemotherapeutic drug, applied in the field of tumor drug preparation, can solve the problems of easy occurrence of chemoresistance, large toxic and side effects, and restriction of clinical application, and achieves the effect of solving the problem of chemoresistance and promoting chemosensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
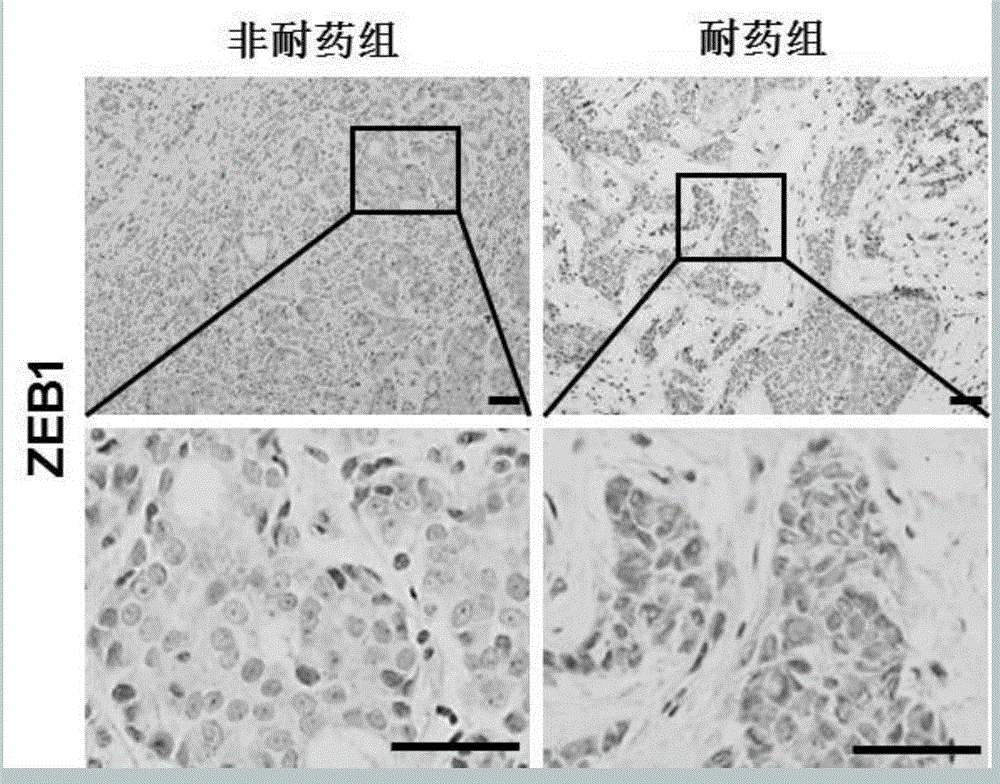
[0054] ZEB1 is closely related to chemotherapy resistance in breast cancer
[0055] (1) Tissue samples of 233 breast cancer cases receiving standard neoadjuvant chemotherapy were collected, and the efficacy of chemotherapy was evaluated using the WHO chemotherapy efficacy evaluation criteria. Among them, 69 were drug-resistant cases and 164 were non-drug-resistant cases. chemical staining;
[0056] (2) Baking slices: first place the tissue slices in a 65°C oven and bake for 2 hours, then take them out and cool to room temperature;
[0057] (3) Prepare the reagents required for dewaxing in the fume hood, put the tissue sections in xylene I for 10 min, xylene II for 10 min, absolute ethanol for I5 min, absolute ethanol II for 5 min, 95% ethanol for 10 min, and 80% ethanol for 10 min, then Wash with TBST on a shaker, 5min, twice, 100rpm;
[0058] (4) Antigen retrieval and 3% H 2 o 2 Remove endogenous hydrogen peroxide;
[0059] (5) Seal the tissue with 5% goat serum at room ...
Embodiment 2
[0067] Construction of stable cell lines of breast cancer overexpressing ZEB1
[0068] (1) Cloning the full-length CDS of the human ZEB1 gene by means of molecular biology;
[0069] (2) PCR amplification and recovery of cloned products;
[0070] (3) Ligate the recovered product to the vector plasmid pLV-EF1-MCS-IRES-Bsd, identify by double enzyme digestion, and sequence to ensure the correctness of the constructed target vector;
[0071] (4) Utilize the Letivirus system to package the target plasmid successfully constructed above, and measure the virus titer;
[0072] (5) The packaged virus was used to transfect human breast cancer cell MDA-MB-231. After 48 hours of transfection, 10 μM BSD was added for stable strain selection, and finally a stable cell line with ZEB1 overexpression was obtained through screening.
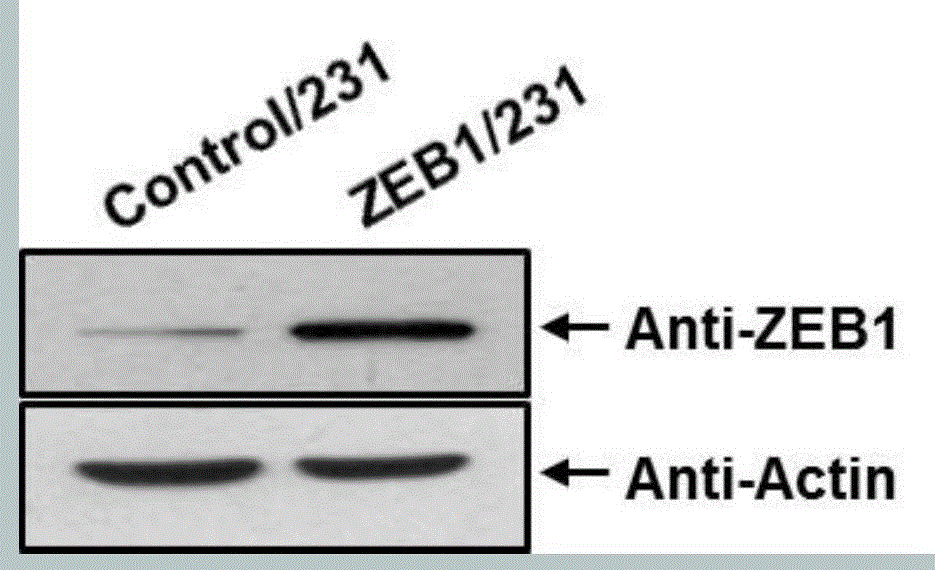
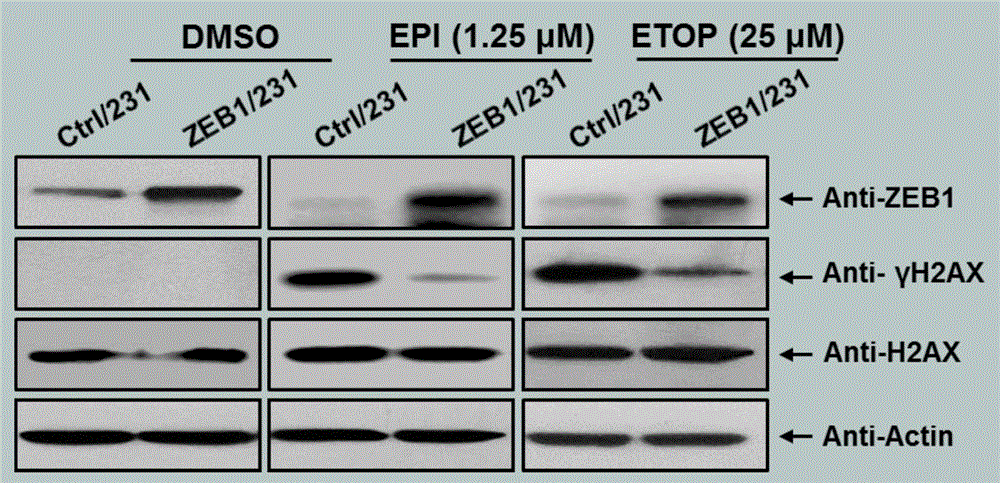
[0073] The overexpression efficiency of ZEB1 was verified by Western blot method ( figure 2 ), the successful construction of this stable cell line provides an...
Embodiment 3
[0075] Preparation of Le-shZEB1
[0076] (1) Design and synthesize ZEB1shRNA sequence online;
[0077] (2) After annealing the ZEB1shRNA sequence (95°C, 5 minutes), ligate it to the vector plasmid pLV-H1-EF1α-puro, and double-enzyme digest to determine whether the target plasmid (BamH1, Sac1) is successfully constructed;
[0078] (3) Use the Letivirus system to package the target plasmid successfully constructed above, and measure the virus titer to construct Le-shZEB1:
[0079] a. Dilute 293T cells at 1.0×10 per well 6 The amount of cells was placed in a 6-well plate, and the cells were transfected when the cell density reached about 90%;
[0080] b. Take a sterile 1.5ml centrifuge tube, add 7.5μl Lipofectamine2000 and 250μl Opti-MEM into it, mix gently, and let stand at room temperature for 5min;
[0081] c. Take another sterile 1.5ml centrifuge tube, add 1.5μg construct pLV vector (purpose plasmid), 1.5μg packaging plasmid (each packaging plasmid 0.5μg, Gag-pol+Rev+VSV-G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com