Method using derivatization HPLC-DAD method to determine small-molecule halogenated carboxylic acid in medicine
A halogenated carboxylic acid and small molecule technology, applied in the field of drug analysis and detection, can solve the problems of inability to suppress the dechlorination reaction, high baseline noise, and unreported system comparison.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] 1.3. Solution preparation
[0043] Each halogenated carboxylic acid stock solution (hereinafter referred to as HCA): take an appropriate amount of HCA, accurately weigh it, place it in a 10mL measuring bottle, dilute to the mark with 70% acetonitrile, and shake well. Each stock solution has good stability within 8 hours at room temperature.
[0044] 2-nitrophenylhydrazine hydrochloride, 3-nitrophenylhydrazine hydrochloride, 4-nitrophenylhydrazine hydrochloride and 2,4-dinitrophenylhydrazine test solution (hereinafter referred to as 2-NPH·HCl, 3-NPH·HCl, 4-NPH·HCl and DNPH): Take about 100 mg of each reagent, weigh it accurately, place it in a 10 mL measuring bottle, dilute to the mark with 80% acetonitrile, and shake well. Must be freshly prepared.
[0045] 1-Ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride solution (hereinafter referred to as EDC·HCl): take 1-ethyl-(3-dimethylaminopropyl) carbodiimide 200mg of amine hydrochloride, accurately weighed, placed ...
Embodiment 1
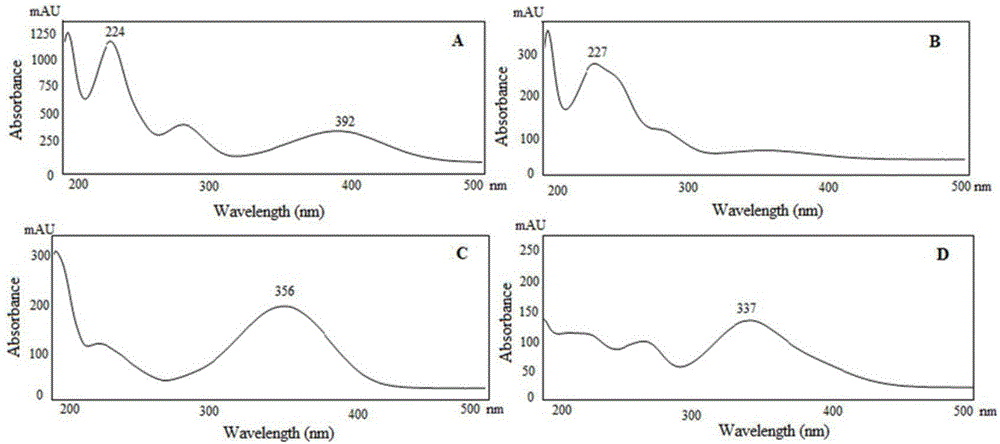
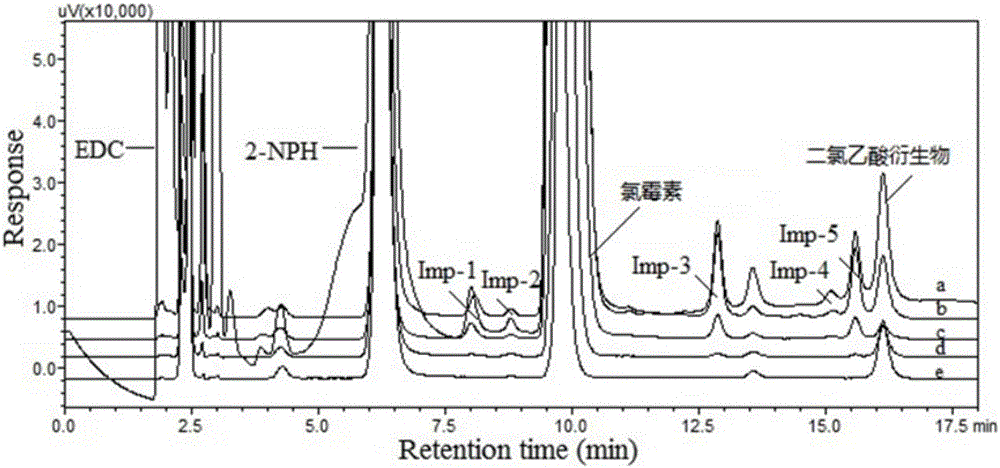
[0049] Taking chloroacetic acid as a representative, it was derivatized with four kinds of nitrophenylhydrazine derivatization reagents at the same concentration. See 1.4 for derivatization experimental conditions. Under the same chromatographic conditions, use DAD to scan each derivatized product, record the spectrum and chromatogram of the product, and evaluate the pros and cons of the derivatized reagent with the maximum absorption wavelength and absorption intensity of the product.
[0050] figure 1It shows that except for 3-nitrophenylhydrazine, the ultraviolet absorption bands of the products generated by the other three derivatization reagents and chloroacetic acid all present a certain red-shift effect, and the maximum absorption wavelengths are 392nm (2-nitrophenylhydrazine) and 356nm ( 4-nitrophenylhydrazine) and 337nm (2,4-dinitrophenylhydrazine). The ideal situation in this experiment is to measure in the near visible light region (400nm), which can minimize back...
Embodiment 2
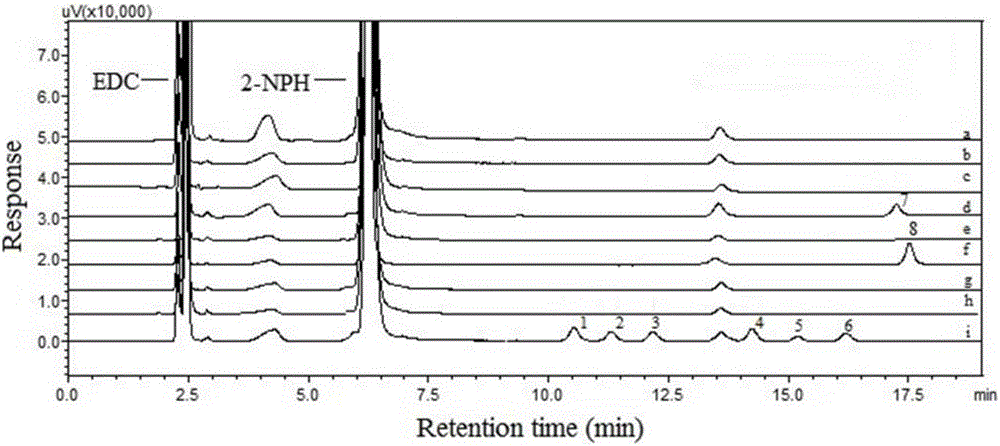
[0053] Preparation of the test solution: Accurately weigh an appropriate amount of the drug, place it in a 5mL volumetric bottle, add 500 μL of derivatization reagent and 500 μL of DC·HCl solution, add 70% acetonitrile to dilute to the mark, vortex for 10 seconds, shake well, and react in the dark for 2 hours , Shake well to obtain the test solution. After filtration, 20 μL was taken for direct injection.
[0054] Preparation of reference solution: Precisely pipette 100 μL of stock solutions of halogenated carboxylic acids with different concentrations, place them in a 5 mL volumetric flask, add 500 μL of derivatization reagent and 500 μL of DC·HCl solution, add 70% acetonitrile to dilute to the mark, vortex for 10 seconds, shake Evenly, avoid light and react for 2h, shake well to obtain the reference substance solution. After filtration, 20 μL was taken for direct injection.
[0055] Chromatographic conditions: Shimadzu LC20AT liquid chromatograph (including online vacuum d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com