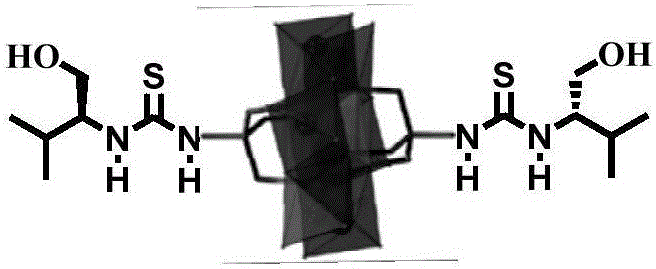
(S)-1-(1-hydroxyethyl-1-isopropyl) thiourea modified Mn-Anderson type heteropoly acid catalyst, and preparation method and application thereof
An isopropyl and heteropolyacid technology, applied in the field of catalytic chemistry, can solve the problems of low activity, difficult recycling and high dosage, and achieve the effects of high catalytic activity, environmental friendliness and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
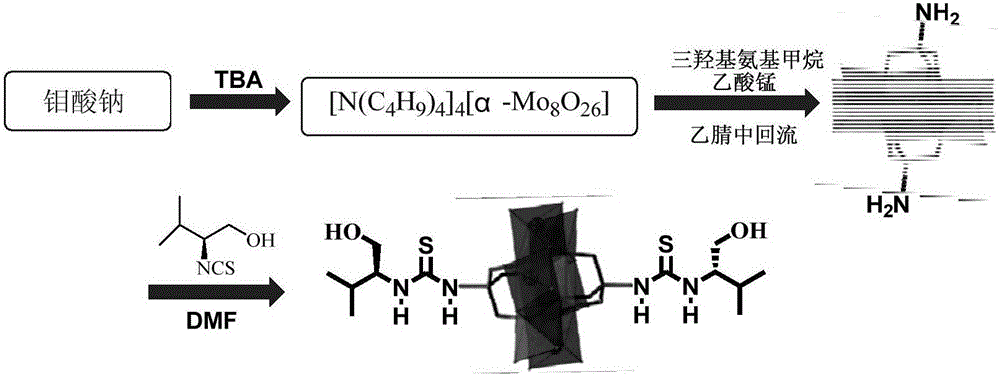
[0034] Polyacid precursor [N(C 4 h 9 ) 4 ] 4 [α-Mo 8 o 26 ] preparation
[0035] In a 50mL flask, Na 2 MoO 4 2H 2 O5.00g (20.7mmol) was dissolved in 12mL of deionized water, and 5.17mL of 6.0N hydrochloric acid solution was added, and vigorously stirred at room temperature for 1-2min. Then 3.34 g (10.4 mmol) of tetrabutylammonium bromide was dissolved in 10 ml of deionized water, and added to the flask under vigorous stirring to form a white precipitate immediately. After the mixture was stirred for 10 minutes, the precipitate was collected on a medium porosity filter by suction and washed with 20 mL of water, 20 mL of ethanol, 20 mL of acetone and 20 mL of diethyl ether, respectively. This crude product (4.78g) was dissolved in 35mL of acetonitrile and allowed to stand at -10°C for 24h. Clear, colorless, block-like crystals were collected by suction filtration and dried under vacuum for 12 hours.
[0036] The clarity of the crystals is lost as they dry. Yield 3.58...
Embodiment 2
[0038] Preparation of (S)-1-(1-hydroxyethyl-1-isopropyl)isothiocyanate
[0039] Add L-valinol (0.7560g, 5mmol) to the dry reaction vessel, dissolve it with 20mL ethanol, then slowly add CS 2 (0.1142g, 15mmol) and triethylamine (0.506mg, 5mmol), after stirring the reaction at room temperature for 1h, then adding di-tert-butyl dicarbonate (Boc 2 O) (1.091mg, 5mmol) and 4-dimethylaminopyridine (DMAP) (18mg, 0.15mmol), after stirring and reacting at room temperature for 2h (gas is generated during the stirring process, attention should be paid to degassing and decompression), which can be obtained 0.7818 g (S)-1-(1-hydroxyethyl-1-isopropyl)isothiocyanate. The yield was 80.9%.
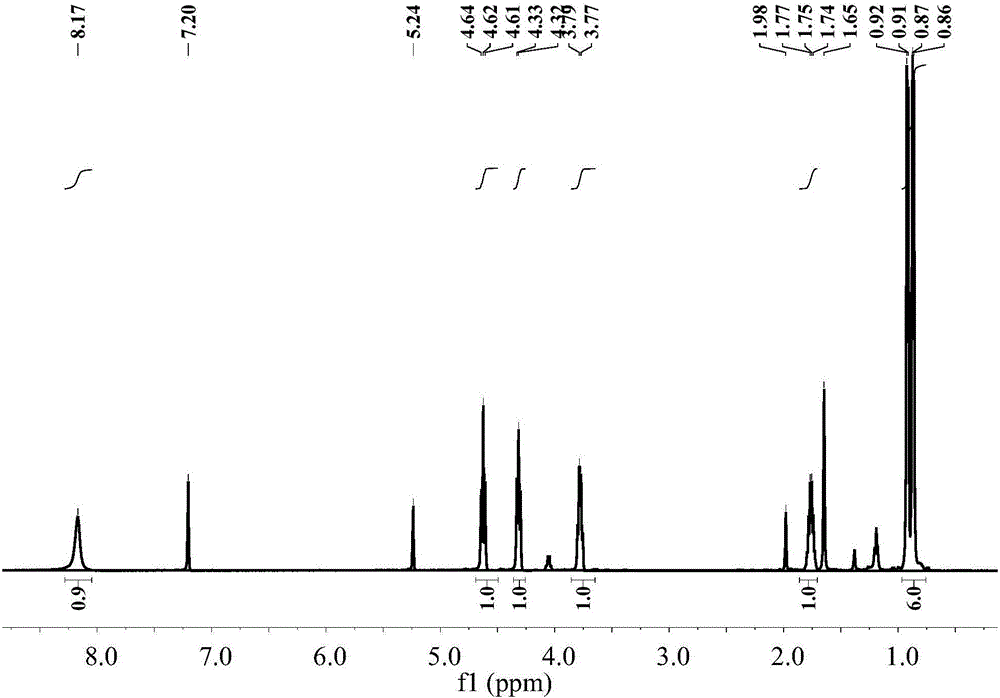
[0040] The NMR spectrum of (S)-1-(1-hydroxyethyl-1-isopropyl)isothiocyanate is shown in image 3 , the specific data are as follows:
[0041] 1 HNMR (501MHz, CDCl 3 )δ8.17(s, 1H), 4.62(t, J=9.1Hz, 1H), 4.32(t, J=7.8Hz, 1H), 3.78(d, J=7.4Hz, 1H), 1.86–1.71( m,1H),0.89(dd,J=24.8,6.7Hz,6H).
Embodiment 3
[0043] Preparation of Mn-Anderson Polyoxometalates Modified by Bilateral Amino Groups
[0044] Take [N(C 4 h 9 ) 4 ] 4 [α-Mo 8 o 26 ] (8.00g, 3.7mmol), Mn(CH 3 COO) 3 2H 2 O (1.49g, 5.6mmol) and (HOCH 2 ) 3 CNH 2 (1.56g, 12.8mmol), in 150mL of acetonitrile solution was refluxed for 16h. The orange solution was cooled to room temperature and filtered to remove a very fine black solid. The filtrate was exposed to ether vapor. After 2 hours, the white precipitate was filtered off. The orange filtrate was again exposed to ether vapor for several days. Plenty of orange crystals were obtained. They were isolated by filtration, washed with acetonitrile and a small amount of ether, and dried under vacuum.
[0045] The infrared spectrum of the Mn-Anderson type polyoxometalate modified with double amino group is as follows Figure 4 shown.
[0046] The nuclear magnetic spectrum of Mn-Anderson type polyoxometalate modified with double amino group is as follows Figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com