A kind of preparation method of dye intermediate 1-naphthol-3,6-disulfonic acid (rg acid)
A dye intermediate, naphthalene disulfonic acid technology, applied in the preparation of sulfonic acid, organic chemistry and other directions, can solve the problems of many by-products, large production pollution, low yield, etc., and achieve mild reaction conditions, reduce pollution, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
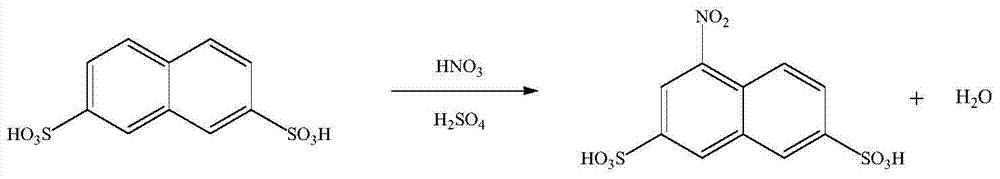
[0032] (1) Nitrification reaction
[0033]
[0034] Add 120 grams of 75% sulfuric acid into a 250ml four-necked bottle, and then put 34g of 2,7-naphthalene disulfonic acid with a solid content of 67.8% into the sulfuric acid, heat to 70°C to dissolve, cool down after complete dissolution, and set the temperature at 0°C Slowly add 7.5 g of concentrated nitric acid with a content of 69.3% dropwise. After the dropwise addition, add 1.38 g of phosphotungstic acid, raise the temperature to 80°C and keep it warm for 2 hours, then slowly add 20g of water dropwise to the reaction solution at 60°C to dilute the reaction solution, so that the final concentration of sulfuric acid reaches about 60%. After dilution, the mixture was cooled to room temperature and allowed to stand for one hour, then suction filtered to obtain 37.6 g of gray-green 1-nitro-3,6-naphthalene disulfonic acid filter cake with a solid content of 65.4% and a yield of 92.3%.
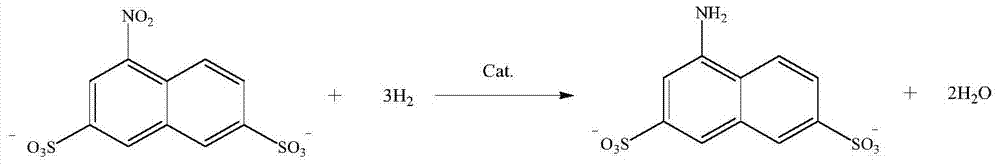
[0035] (2) Reduction reaction
[003...
Embodiment 2
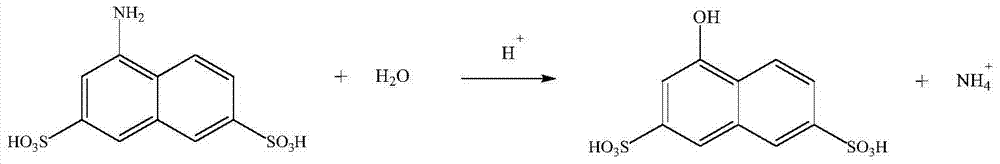
[0042] The difference from Example 1 is that during the hydrolysis reaction, 150g of reducing solution with an amino value of 7.6% was added to a 250ml four-necked bottle and 100g of water was evaporated under reduced pressure, then 120g of 30% hydrochloric acid was quickly added to the concentrated solution, and the temperature was raised to 110 °C, keep the reaction for 10 hours. Liquid chromatographic tracking, when the normalized value of the raw material area is 0.5%, and the product area is greater than 97.2%, the reaction end point is reached. Cool down to room temperature and filter to obtain 13.4 g of gray RG acid filter cake with a content of 81.4%, a purity of 99.2%, and a yield of 95.4%.
Embodiment 3
[0044] (1) Nitrification reaction for mother liquor application
[0045]140 grams of the nitration mother liquor obtained in Example 1 were distilled to 155° C. under reduced pressure at -0.093 MPa. At this time, the concentration of sulfuric acid was about 85%, and then 34 g of 2,7-naphthalene disulfonic acid with a content of 67.8% was dropped into In sulfuric acid, after heating up and dissolving, slowly add 8.3g of concentrated nitric acid with a content of 60.8% dropwise at 20°C, then add 0.7g of phosphotungstic acid, raise the temperature to 85°C and keep it for 4 hours, then slowly drop into the reaction solution at 70°C Add dropwise 28g of water to dilute until the sulfuric acid concentration is about 60%, and filter after cooling down to stand still to obtain 35.9g of nitration filter cake with a content of 68.9% and a yield of 92.8%.
[0046] (2) Reduction reaction
[0047] Take 28.5 g of 1-nitro-3,6-naphthalene disulfonic acid obtained above with a solid content of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com