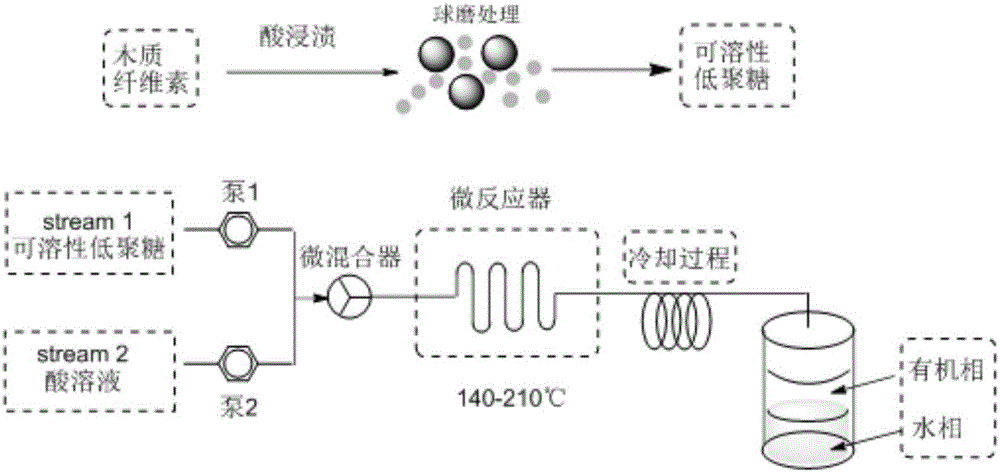
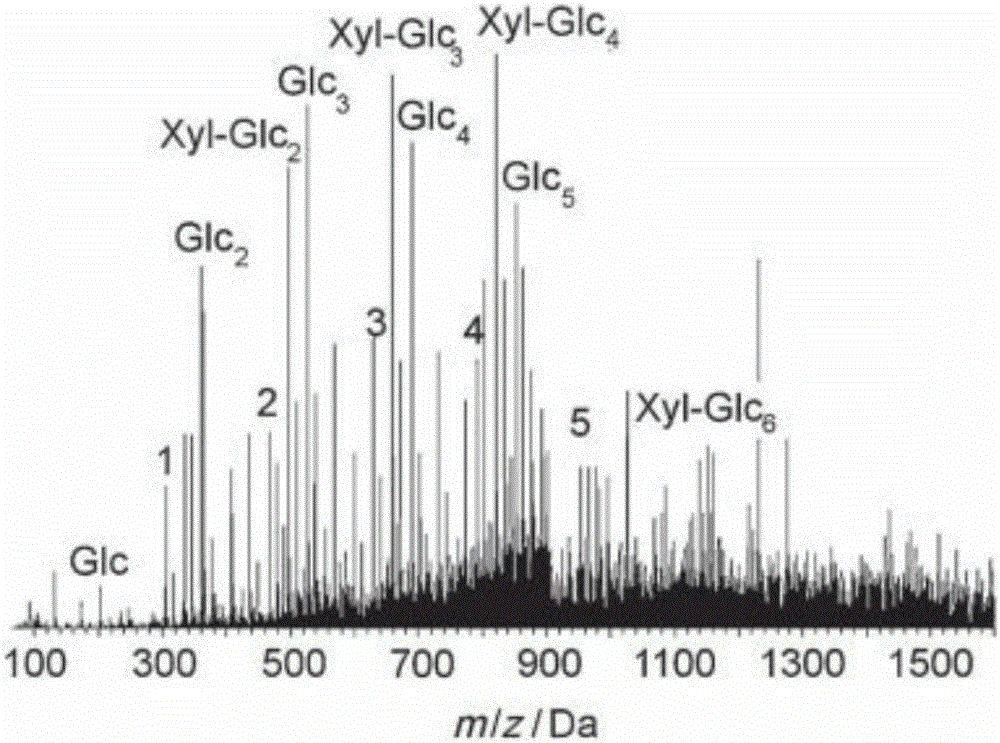
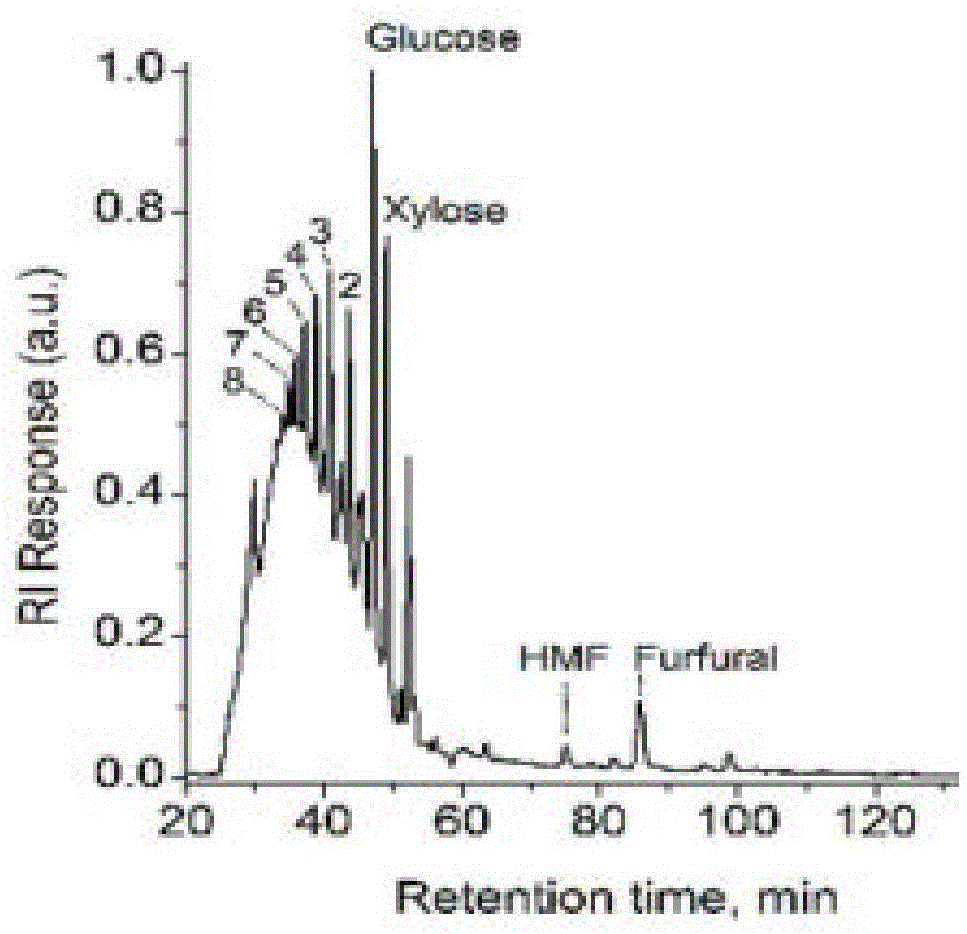
Method for preparing furfural, hydroxymethylfurfural and levulinic acid by means of microchannel reaction device
A technology of hydroxymethylfurfural and microchannel reaction, which is applied in the preparation of carboxylate salts, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as high energy consumption, reduced product yield, and large equipment investment, and achieve energy efficiency. Low energy consumption, improved utilization efficiency, and short processing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Dissolve 9.2 mmol of concentrated sulfuric acid in 100 ml of diethyl ether, take 10.0 g of corn stalks (60 mesh) and disperse them into the diethyl ether sulfuric acid solution, stir at 300 rpm for 1 h, distill off the diethyl ether under reduced pressure. The acid-impregnated corn stalks were put into a ball mill for ball milling (500rpm / min). Note that the temperature should not exceed 45°C for intermittent ball milling. Then the ground corn stalk powder was taken out and weighed to obtain soluble lignocellulose. Take 1g of soluble lignocellulose and disperse it into 10ml of water, shake it for 5 minutes, and filter out the insoluble matter to obtain soluble oligosaccharides and lignin. The yield of soluble oligosaccharides is above 95%.
[0044] The productive rate of soluble oligosaccharides is calculated according to the following formula:
[0045]
[0046] m 0 : the mass of soluble lignocellulose before dissolving;
[0047] m: the mass of the remaining resi...
Embodiment 2
[0050] Dissolve 9.2 mmol of acetic acid in 100 ml of diethyl ether, take 10.0 g of corn stalks (60 mesh) and disperse into the diethyl ether acetic acid solution, stir at 300 rpm for 1 h, distill off the diethyl ether under reduced pressure. The acid-impregnated corn stalks were put into a ball mill for ball milling (500rpm / min). Note that the temperature should not exceed 45°C for intermittent ball milling. Then the ground corn stalk powder was taken out and weighed to obtain soluble lignocellulose. Take 1g of soluble lignocellulose and disperse it into 10ml of water, shake it for 5 minutes, and filter out the insoluble matter to obtain soluble oligosaccharides and lignin. The yield of soluble oligosaccharides is more than 85%.
Embodiment 3~5
[0052] Using the same conditions as in Example 1, the difference is that in Example 3, acetone is used to replace ether; in Example 4, MIBK is used to replace ether; in Example 5, n-butanol is used to replace ether; the yield is shown in Table 1.
[0053] Table 1 embodiment 3~5 detection result
[0054] Example number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com