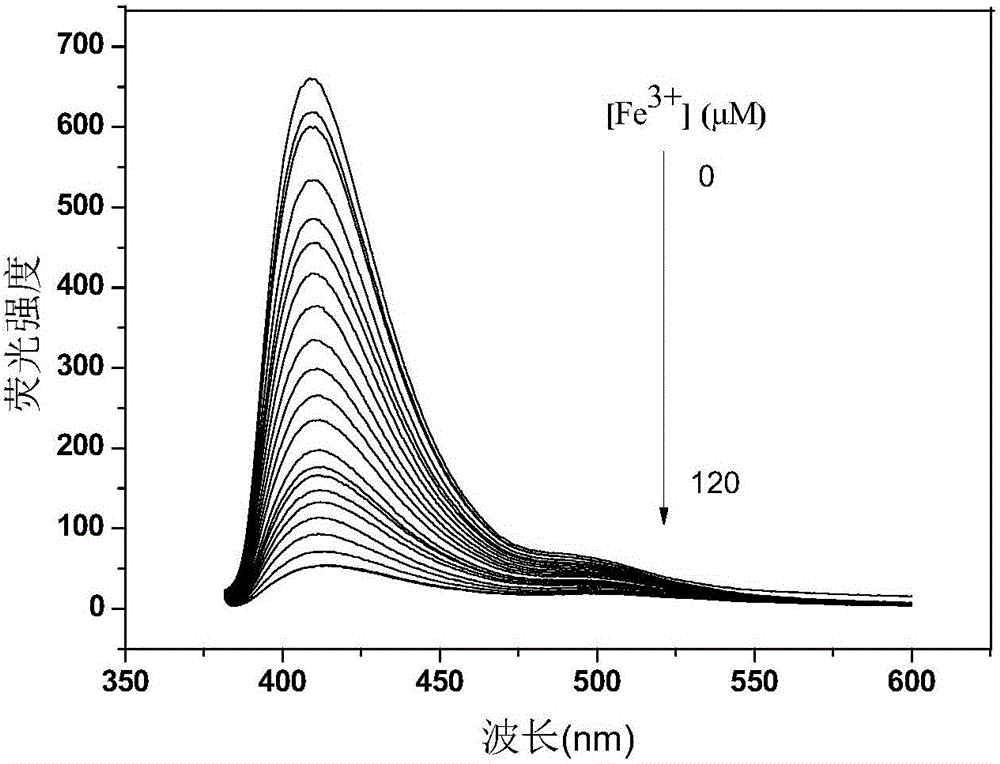
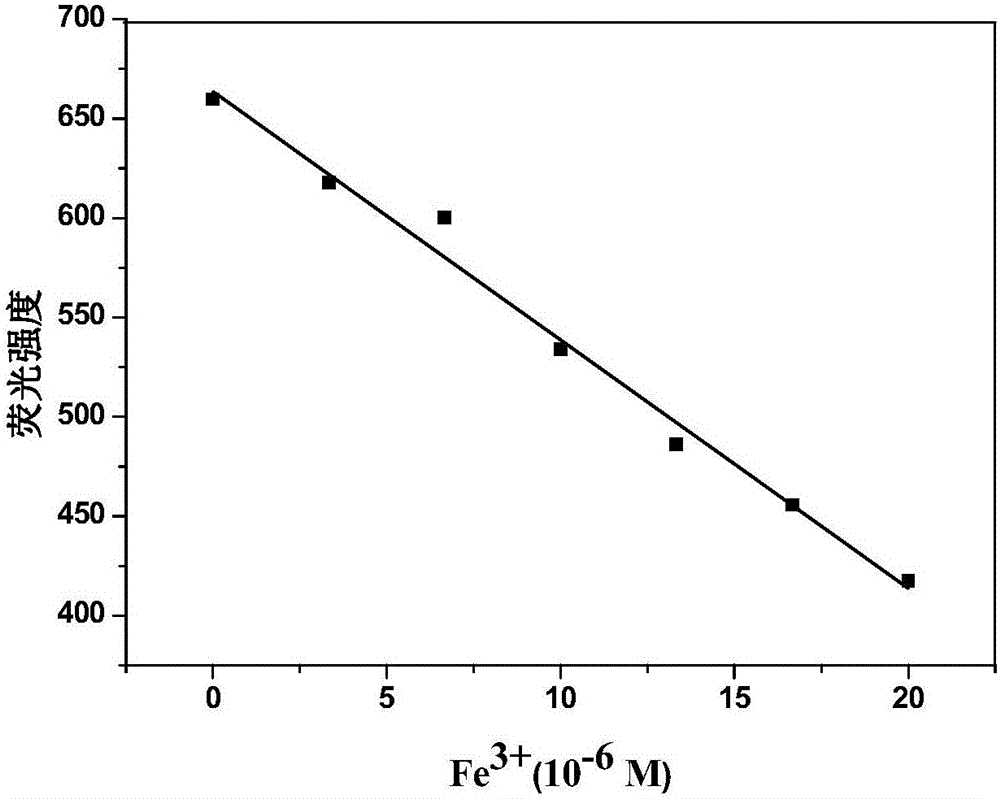
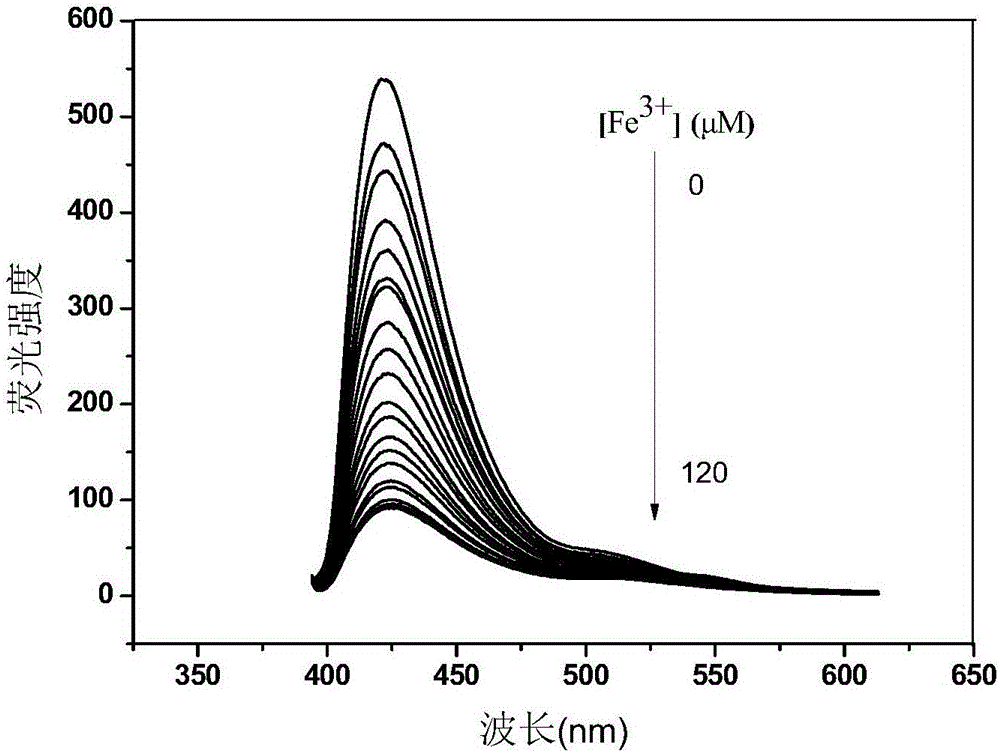
Fe<3+> detection hyperbranched conjugated polymer and preparation method and application thereof
A technology of hyperbranched conjugation and polymers, applied in chemical instruments and methods, measuring devices, fluorescence/phosphorescence, etc., can solve problems such as high production costs, hindering industrial applications, cumbersome synthesis processes, etc., and achieve low detection limits , high selectivity, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] 0.3g (9.08×10 -4 mol) (2E,2'E) diethyl 4,4'-(5-formyl-1,3-phenylene)-bis(but-2-enoic acid ethyl ester) shown in formula I-1 and 62.16mg (4.09×10 -4 mol) 1,8-diazabicycloundec-7-ene (DBU) was dissolved in 10mL of absolute ethanol, and refluxed for 96 hours. After removing ethanol under reduced pressure, the crude product was dissolved with dichloromethane, and then Washing with aqueous hydrochloric acid and deionized water with a pH value of 2, drying with anhydrous magnesium sulfate and removing dichloromethane under reduced pressure, a hyperbranched conjugated polymer (referred to as M1) was obtained with a yield of 75%. The structural characterization results are as follows:
[0026] 1 HNMR (600MHz, CDCl 3 )δ: 10.01 (-CHO), 8.45-6.32 (=CH), 4.32 (-CH 2 ), 1.24 (-CH 3 ); FT-IR (KBr): CH=: 2914; C=C: 3030, 1635; C=O: 1740.
[0027] As tested by gel permeation chromatography, the polymer has Mn=90676, Mw=105664, and Mw / Mn=1.165.
Embodiment 2
[0029]
[0030] 0.3g (9.92×10 -4 mol) (2E,2'E) dimethyl 4,4'-(5-formyl-1,3-phenylene)-bis(but-2-enoic acid ethyl ester) shown in formula I-2 and 67.93mg (4.09×10 -4 mol) DBU was dissolved in 10 mL of absolute ethanol, and refluxed for 96 hours. After the ethanol was removed under reduced pressure, the crude product was dissolved with dichloromethane, then washed with hydrochloric acid aqueous solution and deionized water with a pH value of 2, and then washed with anhydrous After drying over magnesium sulfate and removing methylene chloride under reduced pressure, a hyperbranched conjugated polymer (referred to as M2) was obtained, and its productive rate was 72%, and the structural characterization results were as follows:
[0031] 1 HNMR (600MHz, CDCl 3 ) δ: 8.38-6.43 (=CH), 4.09 (-CH 3 ), 1.26 (-CH 3 ); FT-IR (KBr): CH=: 2908; C=C: 3004, 1651; C=O: 1745.
[0032] As tested by gel permeation chromatography, the polymer has Mn=57283, Mw=66311, and Mw / Mn=1.158.
Embodiment 3
[0034]
[0035] 0.3g (9.08×10 -4 mol) (2E,2'E) diethyl 4,4'-(5-formyl-1,3-phenylene)-bis(but-2-enoic acid ethyl ester) shown in formula I-1 and 62.16mg (4.09×10 -4 mol) DBU was dissolved in 10 mL of absolute ethanol, and refluxed for 96 hours. After the ethanol was removed under reduced pressure, the crude product was dissolved with dichloromethane, then washed with hydrochloric acid aqueous solution and deionized water with a pH value of 2, and then washed with anhydrous After drying over magnesium sulfate and removing dichloromethane under reduced pressure, the resulting product was dissolved in 8 mL of methanol, and then 8 mL of 2 mol / L NaOH aqueous solution was added, hydrolyzed for 12 hours under reflux conditions, cooled to room temperature, and methanol was removed by rotary evaporation to obtain Conjugated polymers (referred to as M3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com