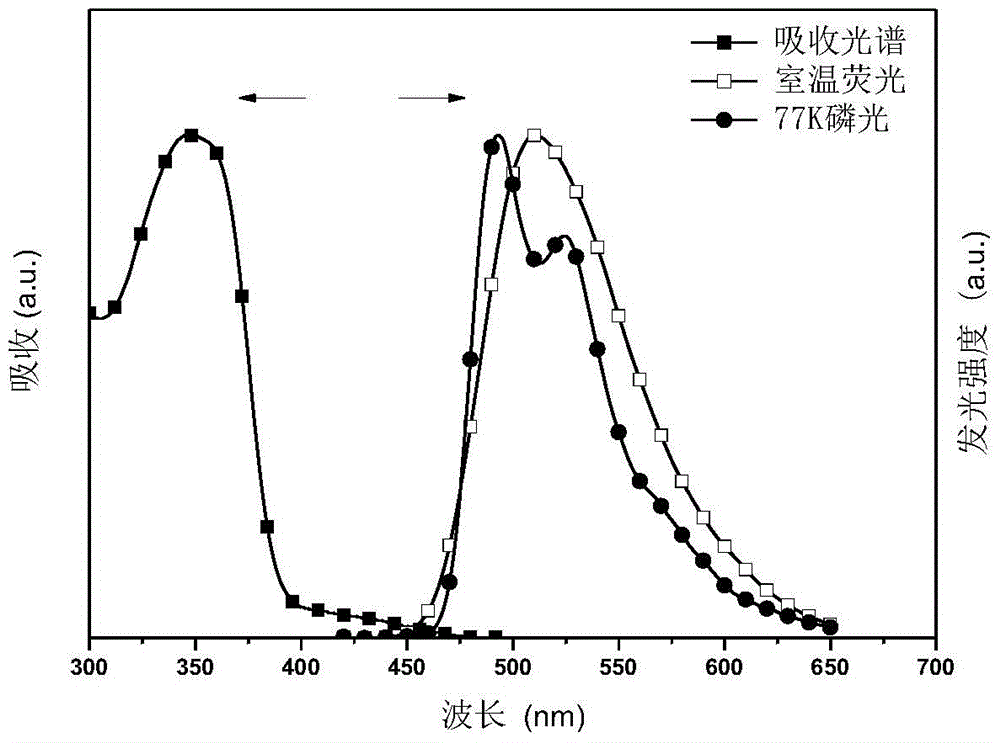
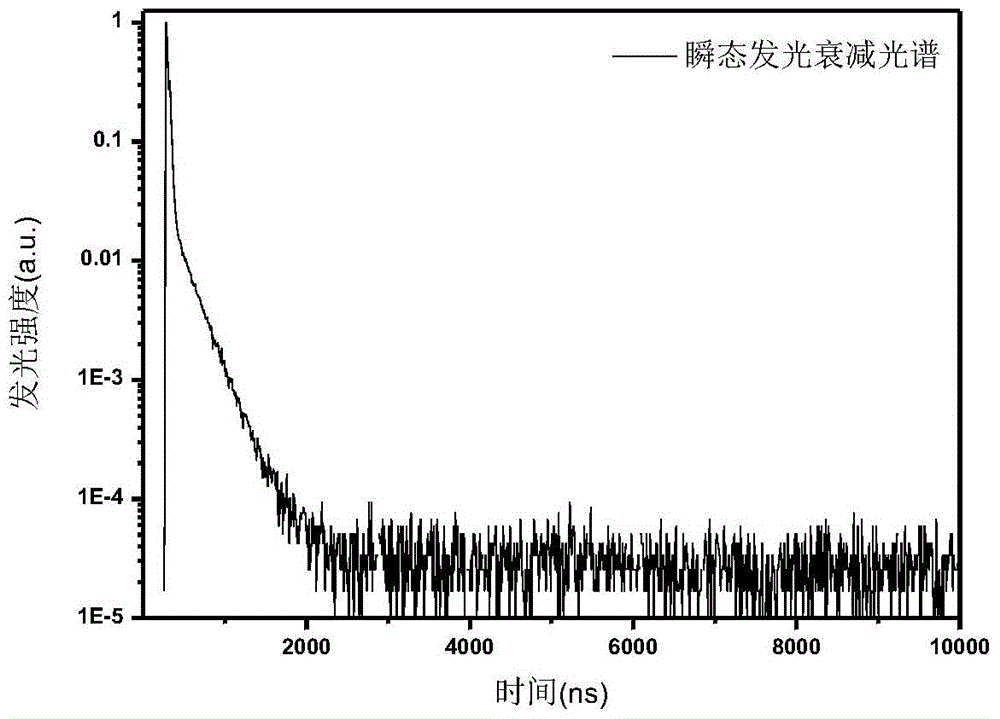
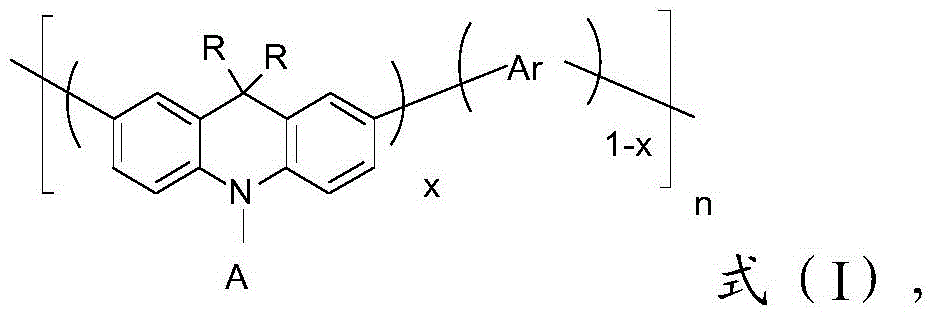
Conjugated polymer and preparation method thereof
A conjugated polymer and polymer technology, applied in the field of conjugated polymers and their preparation, can solve problems such as far from reaching performance, difficulty in delaying fluorescence emission, increasing material cost and difficulty in structural modification, etc., to achieve good luminescence performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The present invention also provides a kind of preparation method of polymer described in the present invention, comprising:
[0067] Copolymerize the compound with the structure of formula (II) and the compound of the structure of formula (III) to obtain the compound of the structure shown in formula (I);
[0068]
[0069] Wherein, R is an alkyl group of C1-C30 or an aryl group of C7-C50;
[0070] Ar is a C6-C50 aryl group or a C4-C50 heteroaryl group;
[0071] A is a C6-C50 aryl group containing an electron-withdrawing group;
[0072] n is 1-200;
[0073] x is changed to 0
[0074] According to the present invention, the present invention will have the compound of formula (II) structure and the compound of formula (III) structure copolymerization, obtain the compound of structure shown in formula (I); Wherein, the compound of formula (II) structure and formula (III) Among the substituents in the compound of structure, the R is preferably a C3-C25 alkyl grou...
Embodiment 1
[0087] Example 1: Synthesis of Polymer PAPhF
[0088] (1) Synthesis of 9,9-dihexyl-9,10-dihydroacridine
[0089] The preparation process is shown in the following formula:
[0090]
[0091] The specific steps are:
[0092] Methyl N-phenyl anthranilate (20.5g, 90mmol) and 80ml of dry tetrahydrofuran were added into a 250ml three-necked flask, the gas was exchanged, and the ether solution of hexylmagnesium bromide (130ml, 2mol / l) dropwise added to the stirred reaction system, the dropwise addition was completed, and stirred at room temperature for 5h; the reaction solution was poured into 200ml water, extracted with water and ether, and the organic phase was rotary evaporated under reduced pressure to obtain the intermediate; the intermediate, 100ml glacial acetic acid Add 30ml of concentrated hydrochloric acid into a 250ml single-necked flask, reflux at 90°C for 24h, cool to room temperature, pour the reaction solution into 200ml of water, extract with water and ethyl ace...
Embodiment 2
[0112] Embodiment 2: the synthesis of polymer PAPTC
[0113] 1) Preparation of unbrominated formula (II) structural compound
[0114] The preparation process is shown in the following formula:
[0115]
[0116] The specific steps are:
[0117] 9,9-dihexyl-9,10-dihydroacridine (2.0 g, 3.7 mmol), 4,6-di-tert-butyl-2-p-bromophenyl- 1,3,5-triazine (1.3g, 3.7mmol), Pd2(dba)3 (0.003g, 0.04mmol), DPPF (0.005g, 0.08mmol), sodium tert-butoxide (0.71g, 7.4mmol) were added Into a 100ml three-neck flask, add 20ml of dry toluene, ventilate, under argon protection, condense at 80°C for 20h, cool to room temperature, extract with water and dichloromethane, rotate the organic phase, and separate by column to obtain 2.0g of the product , 90% yield.
[0118] The obtained product is carried out nuclear magnetic resonance detection, and its hydrogen spectrum is: 1 HNMR (400MHz, CDCl 3)δ8.84(d, J=8.3Hz, 2H), 7.40(d, J=8.3Hz, 2H), 7.30(m, 2H), 6.94–6.81(m, 4H), 6.19(d, J=8.8 Hz, 2H), 2.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com