Fenofibrate-containing nano-liposome as well as preparation method and application thereof
A nano-liposome and fenofibrate technology, applied in the field of medicine, can solve problems such as poor fluidity and dispersibility, increased surface energy of drug powder, and small drug loading, so as to achieve good fluidity and dispersibility, improve Bioavailability, the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of fenofibrate nano liposome
[0043] Accurately weigh fenofibrate, phospholipids and cholesterol, and place them in a 50mL eggplant-shaped bottle. The mass ratio of phospholipids and cholesterol is 9:1, the drug-to-lipid ratio (mass ratio) is 1:10 (the lipid mass is the total mass of phospholipids and cholesterol), and the mass of fenofibrate is 25 mg. Add 3mL of chloroform and shake fully to dissolve the drug and lipid completely. The film was formed by rotary evaporation under reduced pressure at 37° C., and vacuum-dried overnight at room temperature (25° C.), so that the film formed on the bottle wall was evenly dried. Add 6 mL of PBS buffer solution (the specific formula is NaCl137mmol / L, KCl2.7mmol / L, NaCl2.7mmol / L, NaCl2. 2 HPO 4 10mmol / L, KH 2 PO 4 2mmol / L, the PBS buffer concentration is 0.01mmol / L), the hydration temperature is 37°C, and the hydration time is 30min; the probe is ultrasonicated in an ice-water bath to reduce the...
Embodiment 2
[0044] Embodiment 2, the preparation of fenofibrate nano liposome
[0045] Accurately weigh fenofibrate, phospholipids and cholesterol, and place them in a 50mL eggplant-shaped bottle. The mass ratio of phospholipids and cholesterol is 4:1, the drug-to-lipid ratio (mass ratio) is 2:75 (the lipid mass is the total mass of phospholipids and cholesterol), and the mass of fenofibrate is 6 mg. Add 3mL of chloroform and shake fully to dissolve the drug and lipid completely. The film was formed by rotary evaporation under reduced pressure at 37°C, and dried in vacuum at room temperature overnight, so that the film formed on the bottle wall was evenly dried. Add 10mL of PBS buffer solution (the specific formula is NaCl137mmol / L, KCl2.7mmol / L, NaCl2.7mmol / L, NaCl2. 2 HPO 4 10mmol / L, KH 2 PO 4 2mmol / L, PBS buffer concentration is 0.01mmol / L), the hydration temperature is 37°C, and the hydration time is 30min. In an ice-water bath, the probe is ultrasonicated to reduce the particle...
Embodiment 3
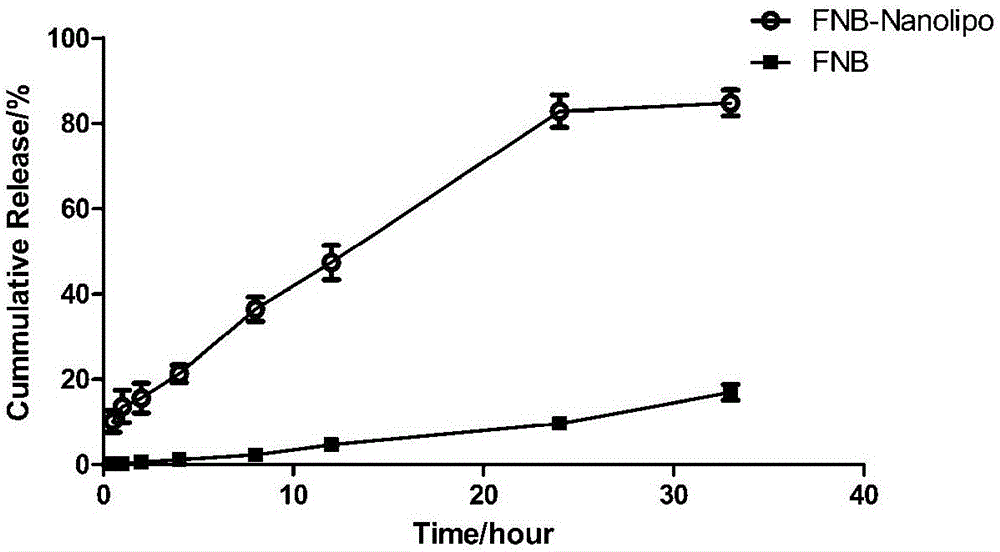
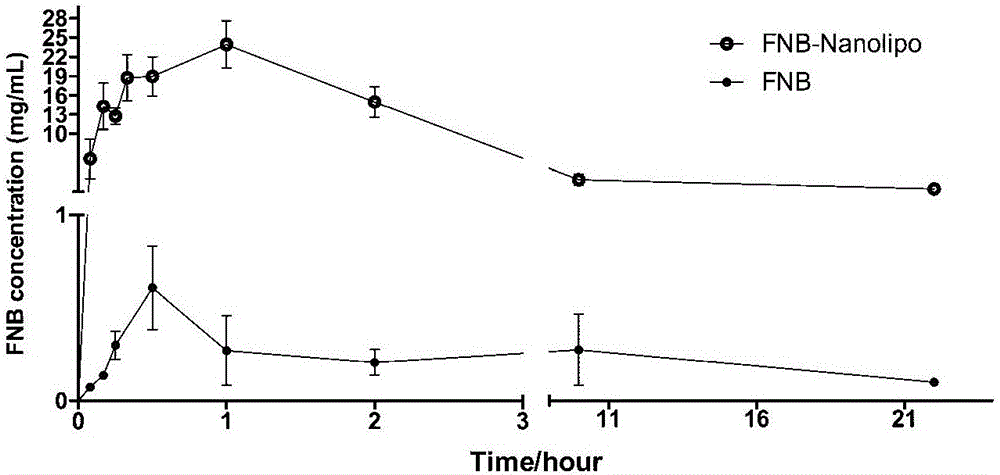
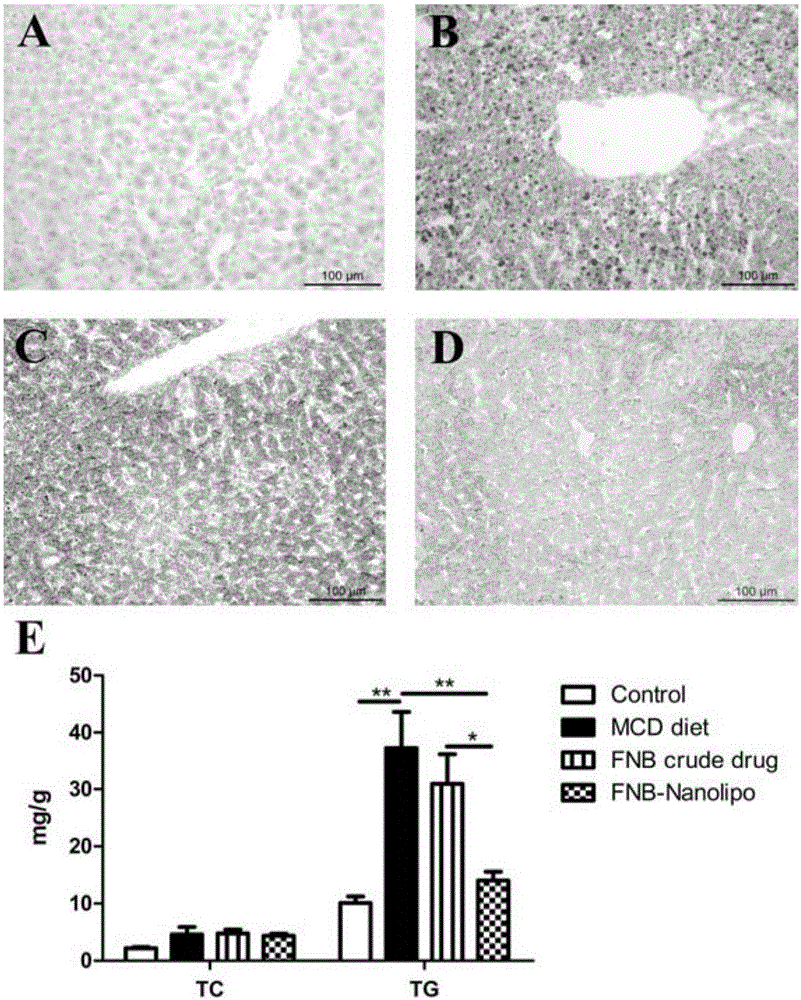
[0046] Embodiment 3, the quality evaluation of fenofibrate nano liposome
[0047] 1. Determination of particle size and Zeta potential: Take the fenofibrate nanoliposome of Example 1 of the present invention, measure it with a Malvern laser particle size analyzer, and record the average particle size, polydispersity index (PDI) and Zeta potential. The average particle size is 122.1±1.40nm, the PDI is 0.293; the Zeta potential is -2.92mV, slightly electronegativity (as shown in Table 1).
[0048] Table 1. Characterization of Fenofibrate Nanoliposomes (n=3)
[0049]
[0050] 2. Determination of encapsulation efficiency and drug loading:
[0051] The method for measuring the encapsulation efficiency of the present invention is an ultracentrifugation method.
[0052] Precision measures the fenofibrate nanoliposome 1mL of embodiment 1, with PBS damping fluid (prescription is NaCl137mmol / L, KCl2.7mmol / L, NaCl2.7mmol / L, NaCl2. 2 HPO 4 10mmol / L, KH 2 PO 4 2mmol / L, the final c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com