A kind of Huang alone element b dispersible tablet and preparation method thereof
A technology of flavonoids and dispersible tablets, applied in the field of medicine, can solve the problems of poor water solubility, reduced inhibitory effect, low cross-resistance, etc., and achieves good stability, increased disintegration rate and dissolution, and less adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
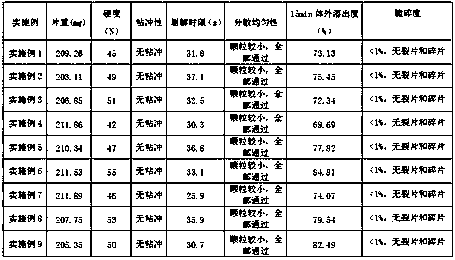
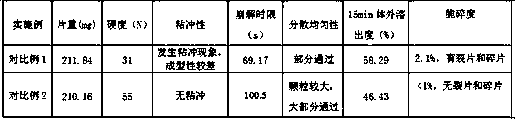
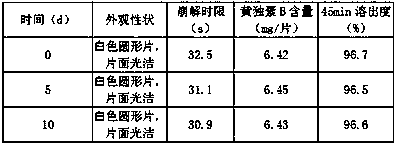
Examples
Embodiment 1
[0022] (1) Weigh 2.5g of lutein B, 20g of microcrystalline cellulose, 70.5g of cellulose lactose, 5.0g of cross-linked sodium hydroxymethyl starch, and 2g of micropowdered silica gel according to the weight ratio, and grind them separately, and pass through 100 meshes sieve, spare;
[0023] (2) Mix 2.5g of luteolin B, 20g of microcrystalline cellulose, 70.5g of cellulose lactose, and 2.5g of cross-linked hydroxymethyl starch sodium in equal increments, add 4g of soft material made of 70% ethanol, and pass Granulate with a 20-mesh sieve, dry in an oven at 55°C to constant weight, pass through a 20-mesh sieve for granulation;
[0024] (3) Then add micropowder silica gel and 2.5g cross-linked sodium hydroxymethyl starch, mix well, and press into tablets to obtain dispersible tablets, each weighing 0.20±0.01g.
Embodiment 2
[0026] (1) Weigh 2.5% of lutein B, 20% of microcrystalline cellulose, 70.5% of cellulose lactose, 5.0% of cross-linked sodium hydroxymethyl starch, and 2% of micropowdered silica gel in proportion by weight, and grind them separately, and pass through 100 Mesh sieve, spare;
[0027] (2) Mix luteolin B, microcrystalline cellulose, cellulose lactose, and 3.75% cross-linked hydroxymethyl starch sodium in equal increments, and add 70% ethanol containing 6% of the total mass of traditional Chinese medicine in step (1). For soft materials, pass through a 20-mesh sieve to granulate, dry in an oven at 60°C until constant weight, pass through a 20-mesh sieve to granulate;
[0028] (3) Then add micropowder silica gel and the remaining 1.25% of cross-linked sodium hydroxymethyl starch, mix well, and press into tablets to obtain dispersible tablets, each weighing 0.20±0.01g.
Embodiment 3
[0030] A yellow sole B dispersible tablet, the raw material composition is: 2.5% yellow sole B, 20% microcrystalline cellulose, 65.5% cellulose lactose, 10% cross-linked sodium hydroxymethyl starch, 2% micropowder silica gel. The internal addition amount of cross-linked hydroxymethyl starch sodium is 6.0%, and the external addition amount is 4.0%.
[0031] The preparation method is the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com