Method for preparing porcine circovirus inactivated vaccine
A porcine circovirus and inactivated vaccine technology, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problem of no effective treatment for porcine circovirus disease, achieve good antigen recovery rate, and high antigen recovery rate , good effect of vaccine safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The cultivation and identification of embodiment 1 virus
[0030] Get the sample of PCV2WH strain and inoculate in the PK-15 cell suspension of fresh digestion simultaneously by 10%, 37 ℃ static culture 24h, after the cell forms monolayer, add appropriate amount 300mmolD-glucosamine to process according to Tischer method (in order to be able to completely Covering the cell monolayer shall prevail), cultured at 37°C for 30min, discarded D-glucosamine, added DMEM maintenance solution containing 2% newborn bovine serum to continue culturing for 48h, harvested the virus, and placed it in a -20°C refrigerator for temporary storage (long-term storage need to be placed at -80°C).
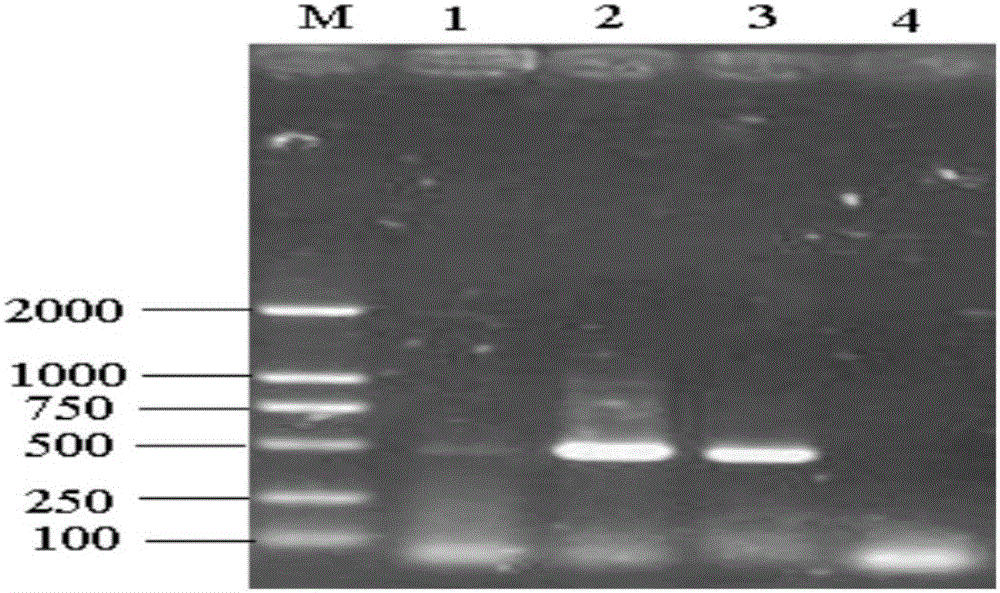
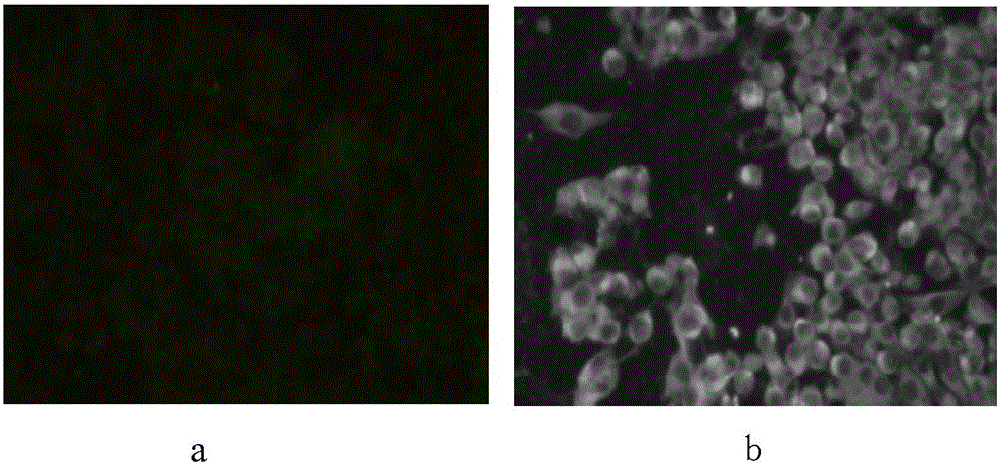
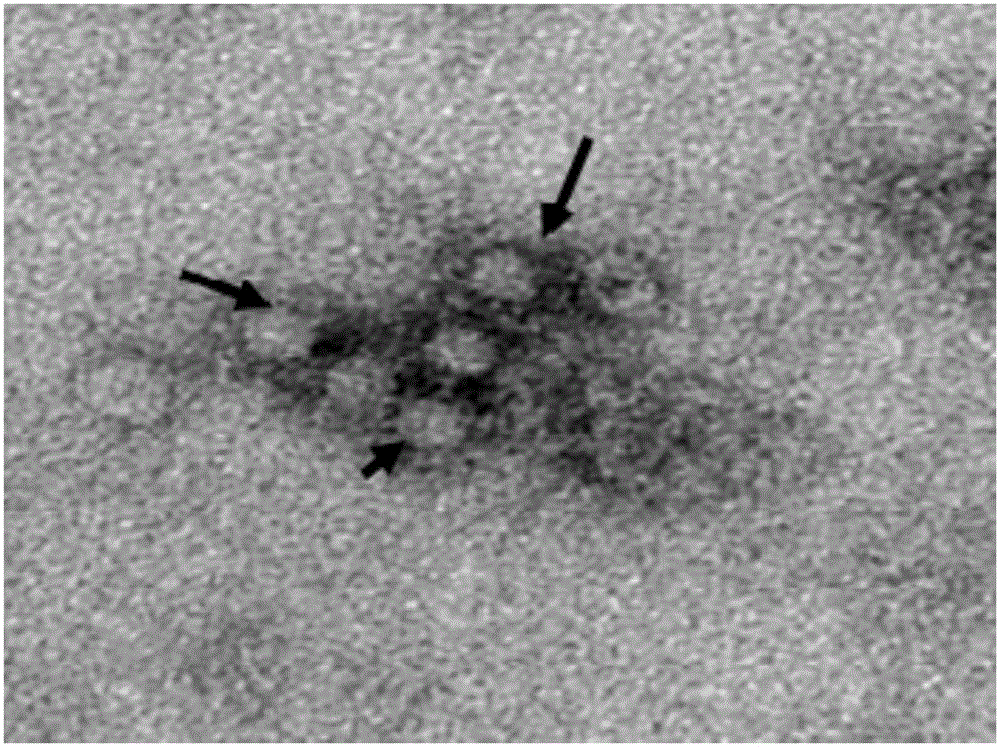
[0031] The identification of the virus is mainly determined by PCR method, indirect immunofluorescence method and electron microscope observation method.
[0032] Referring to the nucleotide sequence of the PCV2 strain (AY122275) published by GenBank, a pair of primers were designed to amplify the ...
Embodiment 2B
[0039] The preparation of embodiment 2BEI inactivator
[0040] BEA and 0.2 mol / L NaOH solution were placed in a water bath at 37°C for 1 hour to prepare a BEI inactivator, and stored at 4°C for future use.
[0041] The inactivation of the formaldehyde of embodiment 3BEI and 2‰ to virus liquid
[0042] Add BEI with a final concentration of 0.002mol / L, 0.001mol / L, 0.0005mol / Ll and 0.00025mol / L and formaldehyde with a concentration of 2‰ to the virus liquid, respectively, at 37°C, 32°C, 27°C and Place it at 22°C for 12 days, and add blocking agent to stop the inactivation. Samples were taken daily and virus levels were determined by indirect immunofluorescence.
[0043] Tables 1 to 4 show the inactivation effect of formaldehyde on PCV2 (WH strain) under different temperature conditions with a concentration of 0.002mol / L, 0.001mol / L, 0.0005mol / L, 0.00025mol / L and a concentration of 2‰.
[0044] Finished product inspection: According to the appendix of "The Veterinary Pharmacopo...
Embodiment 4
[0054] The sub-packaging of the seedlings of the inactivated virus liquid of embodiment 4
[0055] Inactivate BEI and 2‰ formaldehyde in four concentrations (0.002mol / L, 0.001mol / L, 0.0005mol / L, 0.00025mol / L) at different temperatures (37°C, 32°C, 27°C, 22°C) The virus liquid was mixed and subpackaged, and after the sterility test passed, the vaccines mixed with 4 concentrations of BEI and 2‰ formaldehyde inactivated vaccines were compiled into groups A, B, C, D, and E5 (4 kinds at 37°C) Vaccines formulated with concentrations of BEI and 2‰ formaldehyde inactivated are coded as A1, B1, C1, D1, E1, and vaccines formulated with 4 concentrations of BEI and 2‰ formaldehyde inactivated at 32°C are coded as A2, B2, C2, D2, E2, the vaccine obtained under each temperature condition is a group, and so on), and the vaccine is stored at 4°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com