Sustained-release microsphere preparation containing recombinant hepatitis B surface antigen and preparation method thereof
A technology of hepatitis B surface antigen and slow-release microsphere preparations, which is applied in the direction of medical preparations containing active ingredients, antiviral agents, and medical preparations with non-active ingredients. It can solve problems such as affecting immune effects and antigen denaturation, and achieves Effects of inducing cellular immunity, high encapsulation efficiency, and high immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Trehalose and human serum albumin were selected as stability protectors, multiple groups of 0.5 mg / ml HBsAg solutions were taken, trehalose was added to make the concentrations reach 5 mg / ml and 10 mg / ml respectively; human serum albumin ( HSA) and make the concentrations reach 0.5 mg / ml, 1.0 mg / ml, 2.5 mg / ml, 5.0 mg / ml respectively, then freeze-dry in vacuum for 12 hours, and measure the HBsAg antigen activity retention rate by double antibody sandwich ELISA method, the results are shown in the table 1. In Table 1, lyophilization represents vacuum freeze-drying for 12 hours.
Embodiment 2
[0035] Trehalose and human serum albumin were selected as stability protectors, multiple 0.2 ml HBsAg solutions with a concentration of 0.5 mg / ml were taken, and trehalose and human serum albumin were respectively added to obtain trehalose concentrations of 5.0 mg / ml, human Serum albumin concentration of 1.0 mg / ml, 2.5 mg / ml, 5.0 mg / ml, 10.0 mg / ml HBsAg solution, then mixed with dichloromethane, emulsified at 15000 rpm for 150 seconds, centrifuged at 5000 rpm and taken After the supernatant was volatilized with dichloromethane, the HBsAg antigen activity retention rate was measured by double-antibody sandwich ELISA. See Table 1 for test data. The aforementioned emulsification step is represented in Table 1 by emulsification.
[0036] Table 1 Effect of stability protector on the retention rate of antigenic activity of HBsAg solution after lyophilization and emulsification
[0037]
Embodiment 3
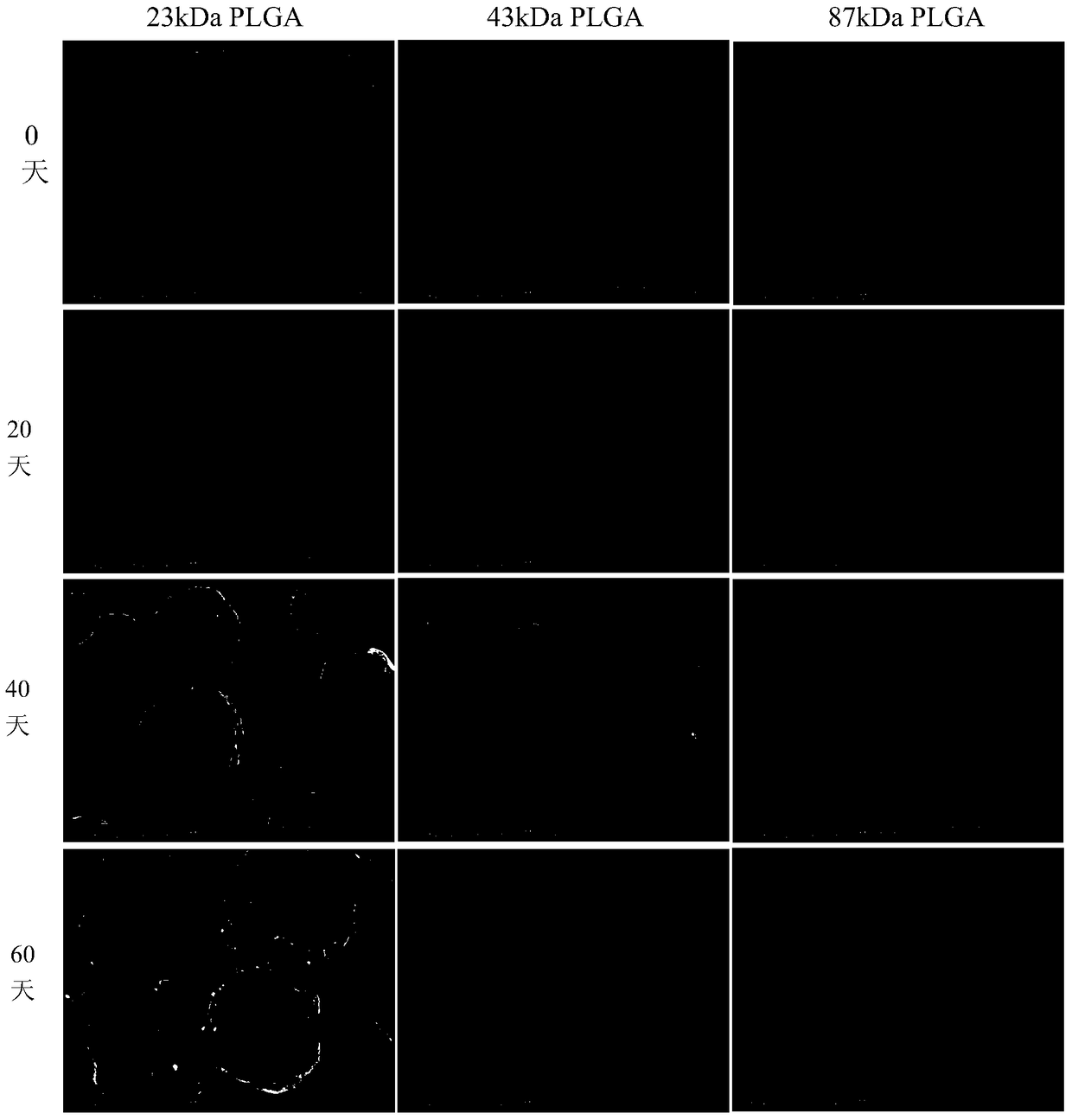
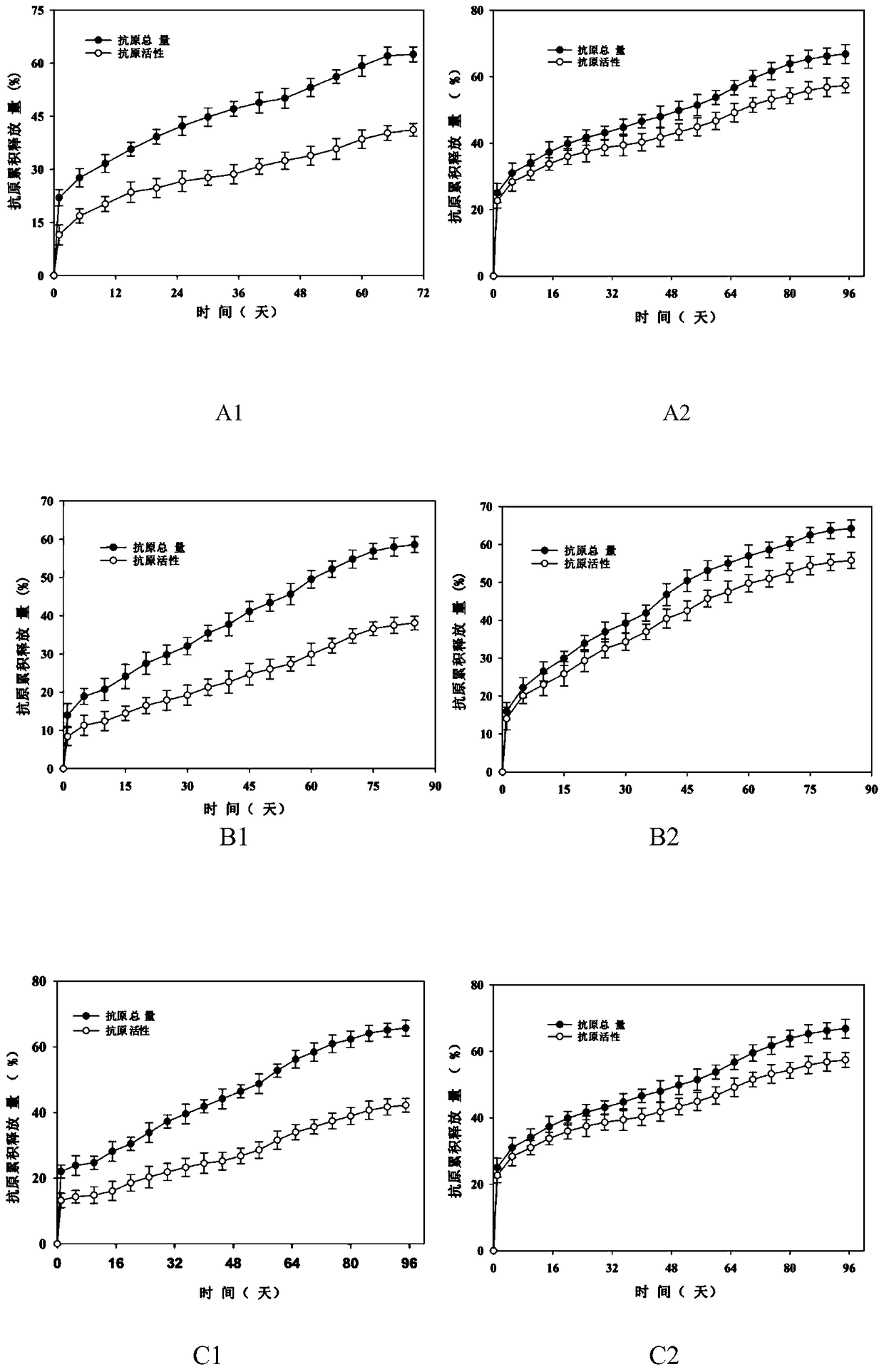
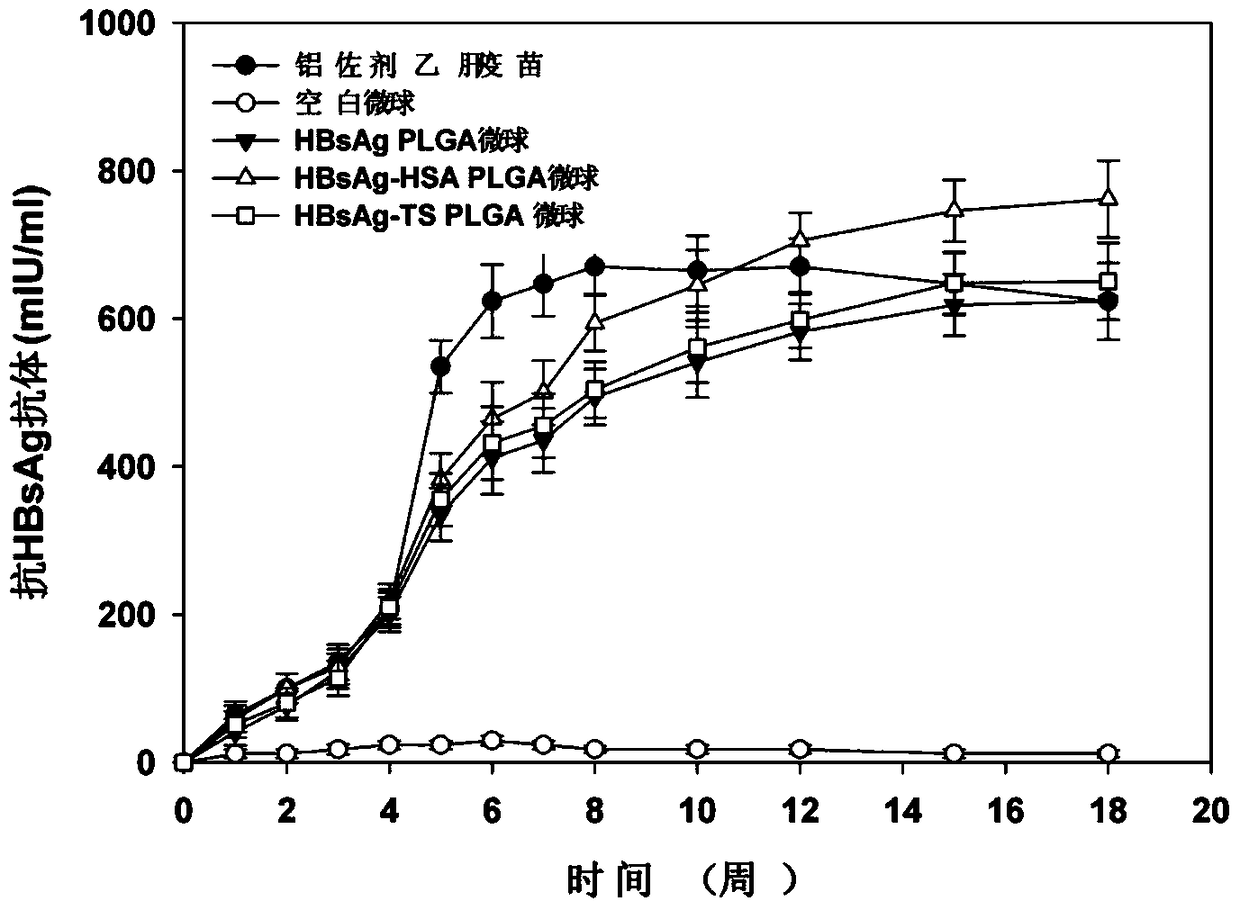
[0039] Human serum albumin (HSA) was used as a stability protector for the preparation of HBsAg sustained-release microspheres, HBsAg and human serum albumin were mixed at a mass ratio of 1:5, and the sodium phosphate solution with a pH value of 5.0 was used as a solvent. The sodium phosphate solution was mixed as the internal aqueous phase. In this mixed solution, the concentration of HBsAg is 1 mg / ml, and the concentration of human serum albumin is 5 mg / ml. Take 100 μl of this solution and add it to 2 ml of dichloromethane and acetone containing 150 mg of 23kDa polylactic acid-polyglycolic acid block copolymer (the mass ratio of polylactic acid to polyglycolic acid (PLA:PLG) is 50:50) (65:35) mixed solution, use a high-speed homogenizer to homogenize it for 300 seconds at a speed of 20,000 rpm to form a stable W / O emulsion, and slowly inject the emulsion into the In 20ml of sodium phosphate (pH5.0) buffer solution containing NaCl and 2.5% PVA, the high-speed homogenizer was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com