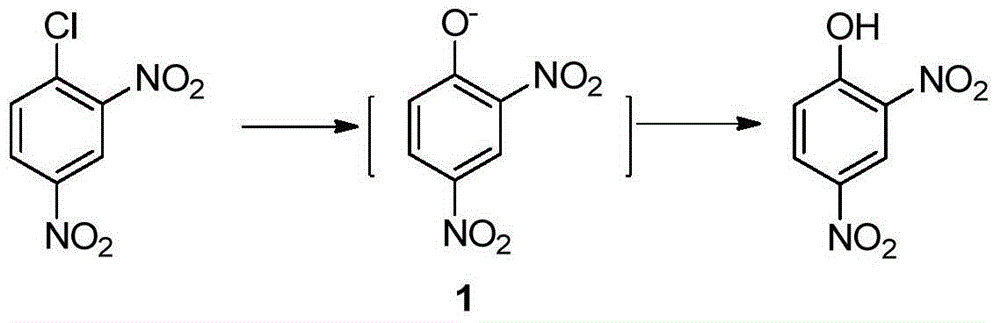
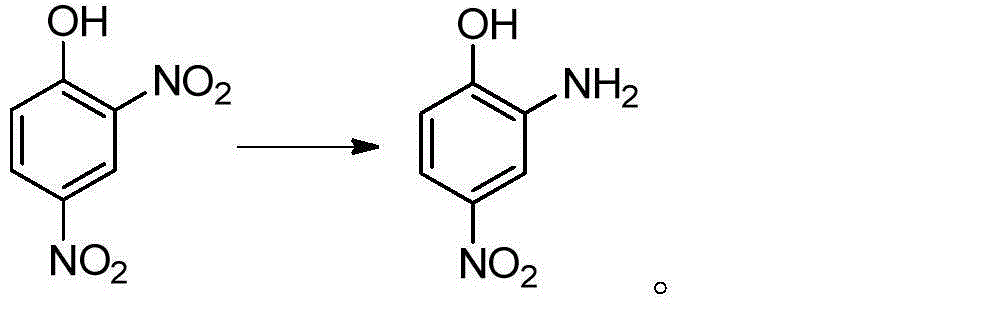
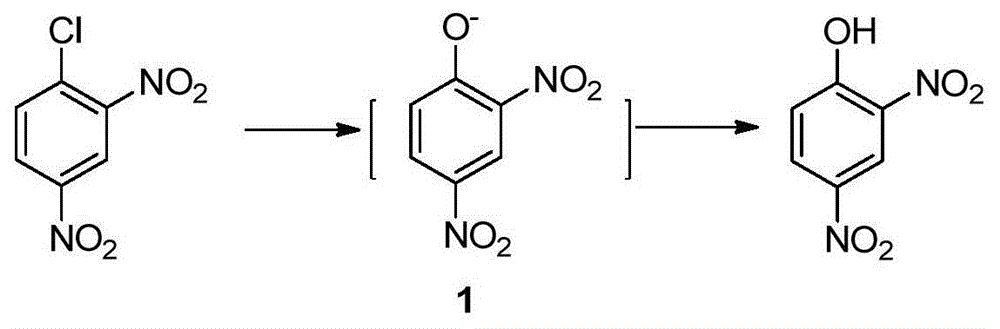
Preparation method of 2-amino-4-nitrophenol
A technology of nitrophenol and dinitrophenol, which is applied in the field of preparation of 2-amino-4-nitrophenol, can solve the problems of being unsuitable for industrial production, cumbersome post-processing operations, and poor atom economy, and achieves no by-products , post-processing is simple, the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 250mL four-neck reaction flask equipped with stirring, reflux condenser and thermometer, add 25mL of water and heat to 60°C; add 15g (0.074mol) of 2,4-dinitrochlorobenzene while stirring, and heat to 90°C; Begin to slowly drip 20.7g mass concentration and be 30% sodium hydroxide solution (the described mass concentration refers to the quality of sodium hydroxide accounts for the percentage of the total mass of sodium hydroxide solution), and the time of dropping remains on 30min~60min approximately, Keep the dropping temperature at 90°C; after adding the lye, keep warm at 95°C and stir the reaction for 1 hour until the oil drops completely disappear (or the content of 2,4-dinitrochlorobenzene as monitored by HPLC is lower than 0.5%;), control the reaction temperature Higher than 100°C; lowered to 70°C, add dropwise HCl with a mass concentration of 15%, in an amount of 20mL-30mL, control the temperature of the system not higher than 85°C during the dropwise addition,...
Embodiment 2
[0040] In a 250mL four-neck reaction flask equipped with stirring, reflux condenser and thermometer, add 25mL of water and heat to 60°C; add 15g (0.074mol) of 2,4-dinitrochlorobenzene while stirring, and heat to 90°C; Begin to slowly drip 21.7g mass concentration and be 30% sodium hydroxide solution (the described mass concentration refers to the percentage that the quality of sodium hydroxide accounts for the total mass of sodium hydroxide solution), keeping the dropping temperature is 92 ℃~94 ℃ , the dropping time is kept at 30min~60min; after adding the lye, keep warm at 95~97°C and stir for 1 hour until the oil drops completely disappear (or the HPLC content of 2,4-dinitrochlorobenzene is lower than 0.5%), Control the reaction temperature not to be higher than 100°C; drop to 70°C, add dropwise HCl with a mass concentration of 36%, in an amount of 5-10mL, control the temperature of the system not higher than 85°C during the dropwise addition, and adjust the pH to Congo red a...
Embodiment 3
[0043] In a 250mL four-neck reaction flask equipped with stirring, reflux condenser and thermometer, add 25mL of water and heat to 60°C; add 15g (0.074mol) of 2,4-dinitrochlorobenzene while stirring, and heat to 94°C; Begin to slowly drip 24.7g mass concentration and be 30% sodium hydroxide solution (the described mass concentration refers to the quality of sodium hydroxide accounts for the percentage of the total mass of sodium hydroxide solution) dropping time remains on 30~60min, keeps dripping The adding temperature is 95°C; after adding the lye dropwise, keep warm at 98-100°C and stir for 30 minutes until the oil drops completely disappear (or the content of 2,4-dinitrochlorobenzene is lower than 0.5% as monitored by HPLC), and the reaction temperature should not be higher than 102°C; drop to 70°C, add dropwise HCl with a mass concentration of 36%, in an amount of 5-10mL, control the temperature of the system not higher than 85°C during the dropwise addition, and adjust th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com