Preparation method of lithium ion battery cathode material NCA with high specific energy
A cathode material and energy technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of poor material performance, non-normal use, and high nickel content, and achieve the effect of improving cycle performance, stable structure, and no sintering process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
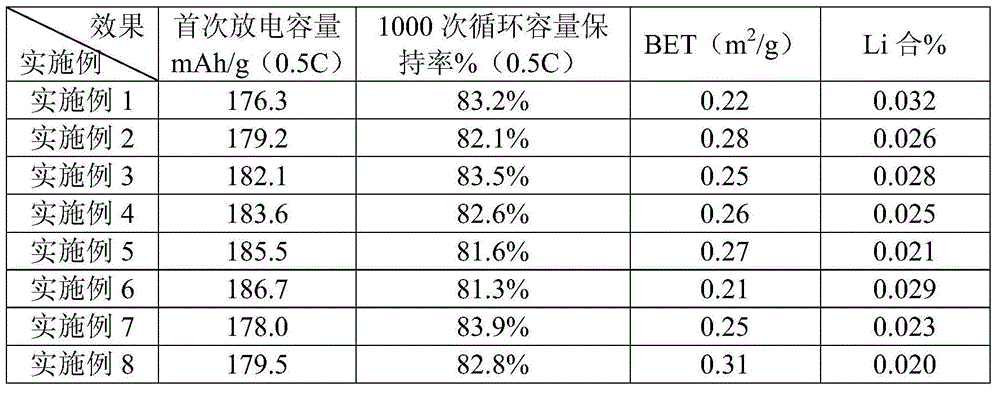
Examples
Embodiment 1
[0017] The preparation method of high specific energy lithium battery cathode material NCA comprises the following steps:
[0018] 1) Mix the nickel sulfate solution, cobalt sulfate solution and aluminum sulfate solution according to the Ni:Co:Al molar ratio of 80:15:5, then add NaOH solution to the solution for neutralization, and produce a ternary system by controlling the crystallization method Hydroxide Ni 0.8 co 0.15 Al 0.05 (OH) 2 precipitation. Then the obtained precipitate was filtered, washed, and dried at 90° C. for 4 hours;
[0019] 2) In the atmosphere furnace, air is introduced, and the dried product obtained in step 1) is heat-treated at 300° C. for 8 hours and then cooled naturally;
[0020] 3) Mix the oxide and lithium hydroxide obtained in step 2) according to the molar ratio of metal cations in the oxide to Li ions of 1:1.02, and after fully mixing, divide the reactants in an air flow or an oxygen flow. Two-stage sintering, first sintering at 450°C for ...
Embodiment 2
[0023] 1) Mix nickel chloride solution, cobalt chloride solution and aluminum chloride solution according to Ni:Co:Al molar ratio of 82:15:3, then add NaOH solution to the solution for neutralization, and produce by controlled crystallization method Ternary hydroxide Ni 0.82 co 0.15 Al 0.03 (OH) 2 The precipitate is then filtered, washed, and dried at 100°C for 5 hours;
[0024] 2) In the atmosphere furnace, air is introduced, and the dried product obtained in step 1) is heat-treated at 350° C. for 9 hours and then cooled naturally;
[0025] 3) Mix the oxide and lithium hydroxide obtained in step 2) according to the molar ratio of metal cations in the oxide to Li ions of 1:1.03, after fully mixing, divide the reactants in the air flow or oxygen flow Two-stage sintering, first sintering at 500°C for 7 hours under air flow, then raising the sintering temperature to 720°C, and then sintering in oxygen flow for 12 hours, and cooling with the furnace after sintering;
[0026] ...
Embodiment 3
[0028] 1) Mix nickel nitrate solution, cobalt nitrate solution and aluminum nitrate solution according to Ni:Co:Al molar ratio of 85:10:5, then add NaOH solution to the solution for neutralization, and produce ternary system by controlled crystallization method Hydroxide Ni 0.85 co 0.1 Al 0.05 (OH)2 precipitation. Then the obtained precipitate was filtered, washed, and dried at 120° C. for 6 hours;
[0029] 2) In the atmosphere furnace, feed oxygen or air, heat-treat the dried product obtained in step 1) at 400° C. for 10 hours, and then cool it naturally;
[0030] 3) The oxide and lithium carbonate obtained in step 2) are mixed according to the molar ratio of metal cations in the oxide to Li ions of 1:1.05, after fully mixing, the reactants are divided into two parts in an air stream or an oxygen stream. Segment sintering, first sintering at 550°C for 6 hours in air flow, then raising the sintering temperature to 760°C, and then sintering in oxygen flow for 25 hours, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com