Calpain inhibitor MDL28170 locally-applied sustained-release membrane capable of promoting repairing of injured spinal cord as well as preparation method and applications of calpain inhibitor MDL28170 locally-applied sustained-release membrane
A technology of MDL28170, a sustained-release film, which is used in medical preparations containing active ingredients, sheet delivery, pharmaceutical formulations, etc., can solve the problems of limited regeneration ability and lack of treatment methods for spinal cord injury, and achieve the effect of avoiding side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
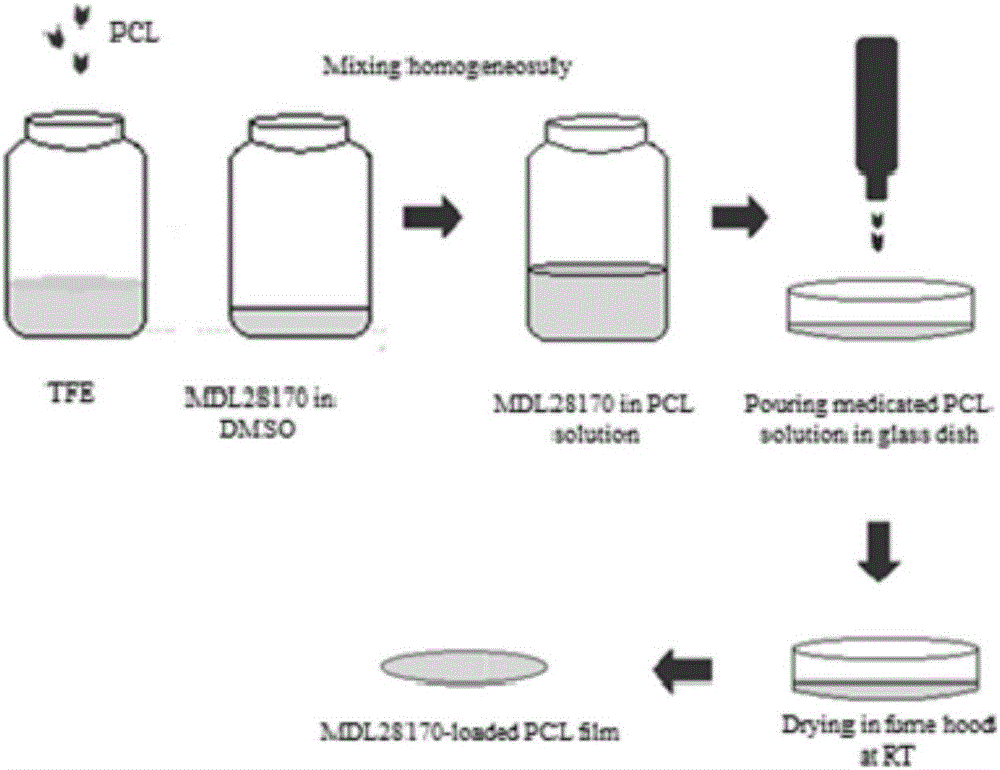
[0029] The calpain inhibitor MDL28170 that promotes the regeneration of spinal cord injury of the present invention is a flexible drug film formed by loading MDL28170 in a slow-release material, and is used to place only on the surface of the damaged spinal cord during spinal cord injury surgery. In order to promote the survival of neurons, it is beneficial to the rehabilitation after spinal cord injury. Among them, MDL28170 is a peptide compound developed for calpain, with a molecular formula of C22H26N2O4 and a molecular weight of 382.45. The structural formula is shown in the following formula.
[0030]
[0031] MDL28170 can significantly reduce the activity of calpain and promote the survival of neurons. The slow-release carrier PCL is a polymer biodegradable biomaterial. The PCL with a molecular weight of 40,000-100,000 is selected. The slow-release film prepared is a flexible sheet. The calpain inhibitor MDL28170 is mixed with the slow-release carrier at a weight per...
Embodiment 2
[0038] Surface Structure Characteristic and Adhesion Performance Detection of PCL Film
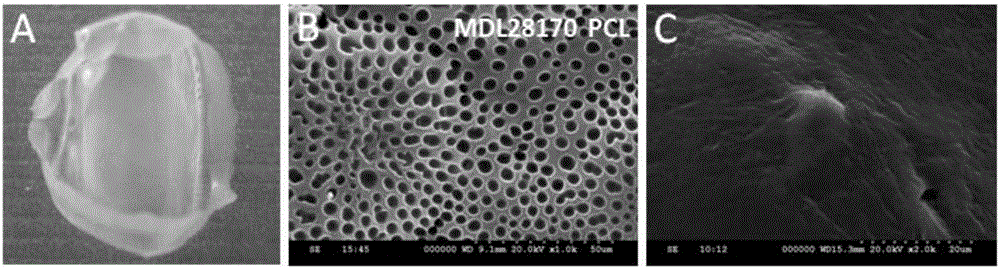
[0039] The detection of the morphological and structural characteristics of the PCL sustained-release film was carried out by scanning electron microscopy (SEM). The material sample is glued on the sample stage, coated with silver glue, and vacuum-sprayed with gold, and the surface structure of the material is observed with a scanning electron microscope. figure 2 Figure A in the middle shows the physical light microscope image of the PCL film. The surface of the PCL film is smooth, translucent, soft, and can be folded and deformed arbitrarily. Figure B shows the scanning electron microscope results of the PCL film loaded with MDL28170. The surface of the PCL film is smooth and has a porous structure. The diameters of the holes are different in micron level, which is conducive to the immersion of interstitial fluid and the release of drugs. Panel C shows that VSC4.1 motoneurons grow adh...
Embodiment 3
[0041] Determination of slow-release properties of PCL membrane by BSA method
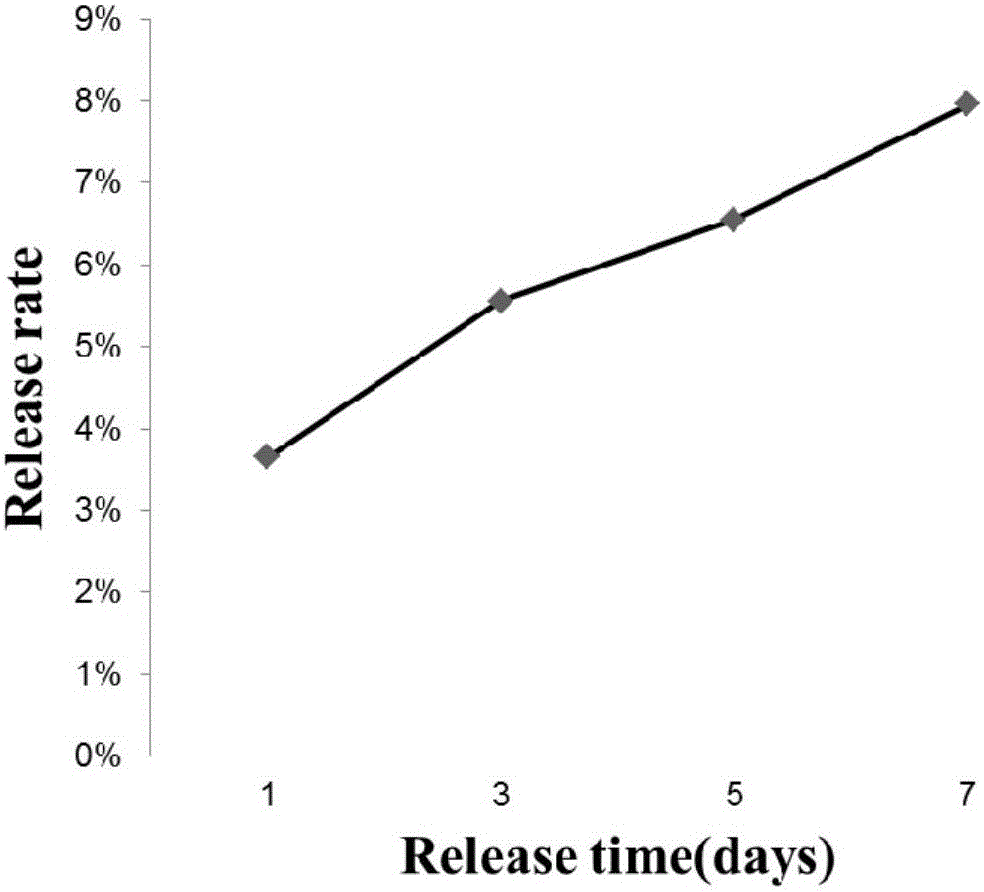
[0042]Take 0.5g of BSA (bovine serum albumin) and dissolve it in 2ml of 0.1M phosphate buffered saline (PBS) to prepare a mother solution with a concentration of 250mg / ml. PCL was dissolved in the organic solvent trifluoroethanol (TFE) at 10% (W / V), vortexed and shaken intermittently to promote the dissolution of PCL, and the PCL&TFE solution was obtained after dissolving and mixing. Take 80μl 250mg / ml BSA mother solution and add it to the PCL&TFE solution, and dilute the concentration to 10mg / ml. Vortex intermittently and shake on a shaker. When BSA is evenly dispersed in the PCL&TFE solution, add the solution to a 6-well plate at 500 μl per well, dry it in a fume hood, gently peel it off with tweezers, and sterilize (75% Soak in alcohol for 10 minutes, wash with sterile 0.1M PBS three times, and dry in a biological safety cabinet for later use). When testing, place the membrane in a 6-well plat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com