Cobalt-doped gel and preparation method thereof
A gel, wet gel technology, applied in glass manufacturing equipment, manufacturing tools, glass molding, etc., can solve problems such as unreported, achieve convenient preparation process, shorten gelation time, reduce chemical stability and The effect of mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
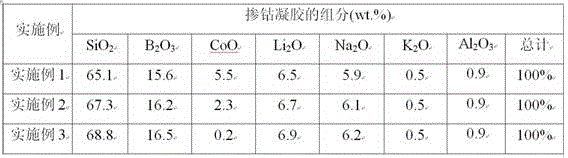
Embodiment 1
[0032] This embodiment includes the following steps:
[0033] (1) Add 90mL of absolute ethanol to a round bottom flask, add 120mL of tetraethyl orthosilicate and 60mL of tributyl borate into the ethanol solvent, add 20mL of deionized water, add hydrochloric acid dropwise to adjust the pH of the solution to 3~5, and Hydrolyze in 60 ℃ constant temperature oil bath stirring pot 7h, stirring rate is 220r / min, obtain about 290ml silicon boron sol;
[0034] (2) At room temperature, 22.20g LiAc·2H 2 O, 12.80gNaAc·3H 2 O, 0.47gKAc, 3.20gAlNO 3 9H 2 O and 0.40gCo(NO 3 ) 2 ·6H 2 O was stirred and dissolved in 80mL deionized water, filtered to obtain about 110ml salt solution; the mol ratio of the obtained salt solution was aluminum nitrate: lithium acetate: sodium acetate: potassium acetate: cobalt salt=43:1088:470:24:182;
[0035] (3) First add the partially hydrolyzed silicon boron sol prepared in step (1) into 60 mL of ethanol, then add the salt solution prepared in step (2) i...
Embodiment 2
[0038] This embodiment includes the following steps:
[0039] (1) Add 45mL of absolute ethanol to a round bottom flask, add 60mL of tetraethyl orthosilicate and 30mL of tributyl borate into the ethanol solvent, add 10mL of deionized water, add hydrochloric acid dropwise to adjust the pH of the solution to 3~5, and Hydrolyze in 60 ℃ constant temperature oil bath stirring pot for 6h, stirring rate is 220r / min, obtain about 145ml silicon boron sol;
[0040] (2) At room temperature, 11.10g LiAc·2H 2 O, 6.40gNaAc·3H 2 O, 0.24gKAc, 1.60gAlNO 3 9H 2 O and 2.05gCoSO 4 ·7H 2 O was stirred and dissolved in 40mL deionized water, filtered to obtain about 55ml salt solution; the mol ratio of the obtained salt solution was aluminum nitrate: lithium acetate: sodium acetate: potassium acetate: cobalt salt=43:1088:470:24:73;
[0041] (3) First add the partially hydrolyzed silicon boron sol prepared in step (1) into 30 mL of ethanol, then add the salt solution prepared in step (2) to the ...
Embodiment 3
[0044] This embodiment includes the following steps:
[0045] (1) Add 90mL of absolute ethanol to a round bottom flask, add 120mL of tetraethyl orthosilicate and 60mL of tributyl borate into the ethanol solvent, add 20mL of deionized water, add hydrochloric acid dropwise to adjust the pH of the solution to 3~5, and Hydrolyze in 60 ℃ constant temperature oil bath stirring pot 7h, stirring rate is 220r / min, obtain about 290ml silicon boron sol;
[0046] (2) At room temperature, 22.20g LiAc·2H 2 O, 12.80gNaAc·3H 2 O, 0.47gKAc, 3.20gAlNO 3 9H 2 O and 4.54gCoAc 2 4H 2 O was stirred and dissolved in 80mL deionized water, filtered to obtain about 110ml salt solution; the mol ratio of the obtained salt solution was aluminum nitrate: lithium acetate: sodium acetate: potassium acetate: cobalt salt=43:1088:470:24:7;
[0047] (3) First add the partially hydrolyzed silicon boron sol prepared in step (1) into 60 mL of ethanol, then add the salt solution prepared in step (2) into the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com