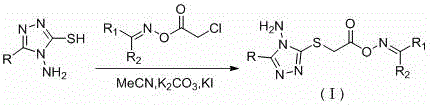
1,2,4-triazole compound containing oxime carboxylate, and preparation method and application thereof
A compound, carboxylic acid oxime technology, applied in the direction of botany equipment and methods, applications, chemicals for biological control, etc., to achieve good biological activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
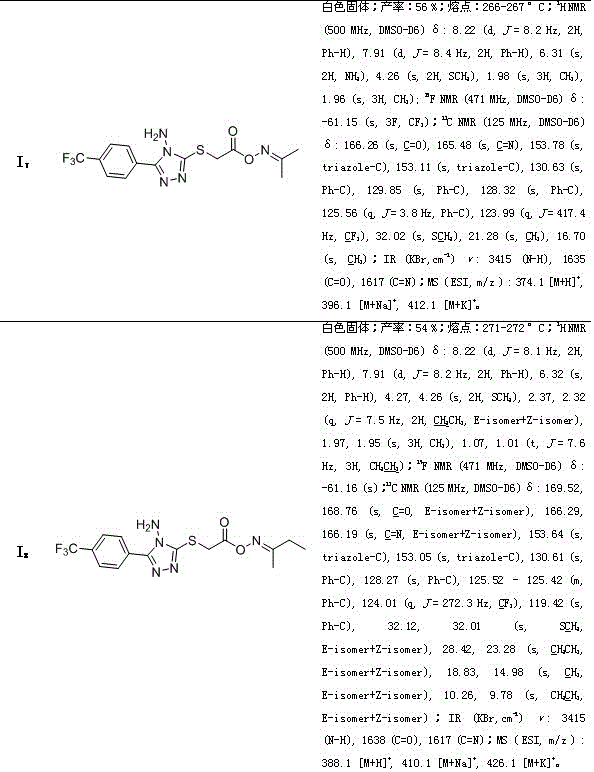
[0055] Embodiment 1: the synthesis of propane-2-ketone O-(2-(3-phenyl-4-amino-1,2,4-triazole-5-thio)acetyl) oxime (compound number is I 1 )
[0056] (1) Dissolve 5.81 g (100.00 mmol) of anhydrous acetone in 100 mL of absolute ethanol, add 20.73 g (150.00 mmol) of anhydrous potassium carbonate and 10.42 g (150.00 mmol) of hydroxylamine hydrochloride, and stir at room temperature for 8 hours. After the reaction, the solvent was evaporated under reduced pressure, washed with water, and dried to obtain 6.15 g of white needle-like crystals, with a yield of 84%.
[0057] (2) Dissolve 7.31 g (100.00 mmol) of acetone oxime in 70 mL of anhydrous acetone, add 15.20 g (110.00 mmol) of anhydrous potassium carbonate, and slowly drop 12.42 g of chloroacetyl chloride diluted with anhydrous acetone in an ice bath. g (110.00mmol), continue stirring for about 2 hours after the dropwise addition, pour the reaction solution into 150 mL of water, separate the liquid, filter, and wash with water t...
Embodiment 2
[0063] Example 2: (Z, E)-butane-2-one O-(2-(3-phenyl-4-amino-1,2,4-triazole-5-thio)acetyl)oxime Synthesis (compound number is I 2 )
[0064] (1) Synthesized under the conditions and method of (1) in Example 1, except that 7.21 g (100.00 mmol) of butanone was added.
[0065](2) Synthesized under the conditions and methods of (2) in Example 1, except that 8.71 g (100.00 mmol) of butanone oxime was added.
[0066] (3) Synthesis under the conditions and methods of (3) in Example 1.
[0067] (4) Synthesize as in the conditions and methods of (4) in Example 1.
[0068] (5) Synthesize as in the conditions and methods of (5) in Example 1.
[0069] (6) Synthesize as in the conditions and methods of (6) in Example 1.
[0070] (7) Synthesized under the same conditions and methods as in (7) in Example 1, except that 0.36 g (2.20 mmol) of (Z,E)-butan-2-one O-(2-chloroacetyl)oxime was added.
Embodiment 3
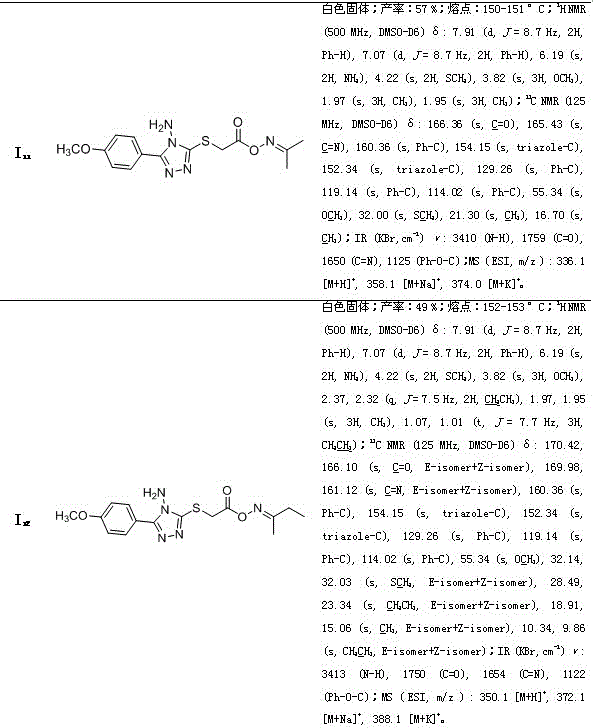
[0071] Embodiment 3: the synthesis of propane-2-ketone O-(2-(3-(4-fluorophenyl)-4-amino-1,2,4-triazole-5-thio)acetyl)oxime ( The compound number is I 3 )
[0072] (1) Synthesize as in the conditions and methods of (1) in Example 1.
[0073] (2) Synthesis under the conditions and methods of (2) in Example 1.
[0074] (3) Synthesized under the conditions and methods of (3) in Example 1, except that 7.01 g (50.00 mmol) of 4-fluorobenzoic acid was added.
[0075] (4) Synthesized according to the conditions and methods of (4) in Example 1, except that 6.73 g (40.00 mmol) of ethyl 4-fluorobenzoate was added.
[0076] (5) Synthesized according to the conditions and methods of (5) in Example 1, except that 4.62 g (30.00 mmol) of 4-fluorobenzohydrazide was added.
[0077] (6) Synthesized under the same conditions and methods as in (6) in Example 1, except that 3.92 g (20.00 mmol) of 2-(4-fluorophenyl)-5-mercapto-1,3,4-oxadiazole was added.
[0078] (7) Synthesized as in Example 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com