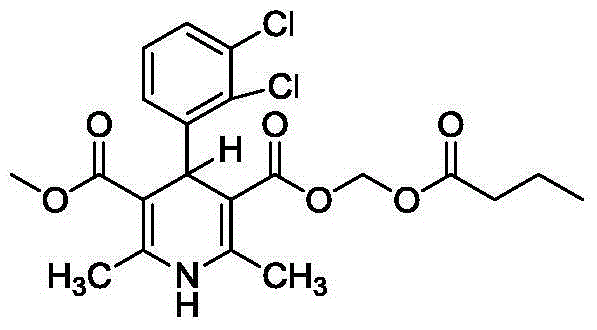
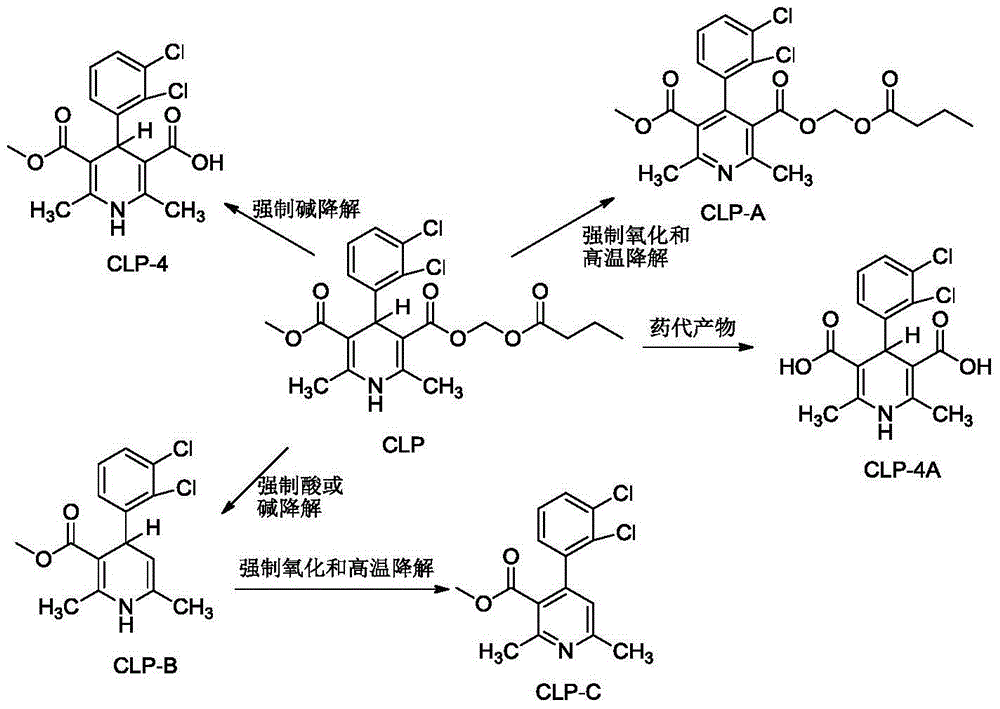
Method for preparing clevidipine fat emulsion injection
A technology of clevidipine and fat emulsion, which is applied in the field of pharmaceutical preparations, can solve problems such as poor stability, difficulty in shearing and dissolving phospholipid oil phase, and impurities in the finished product are easy to exceed the standard, and achieve the effect of small changes in product impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
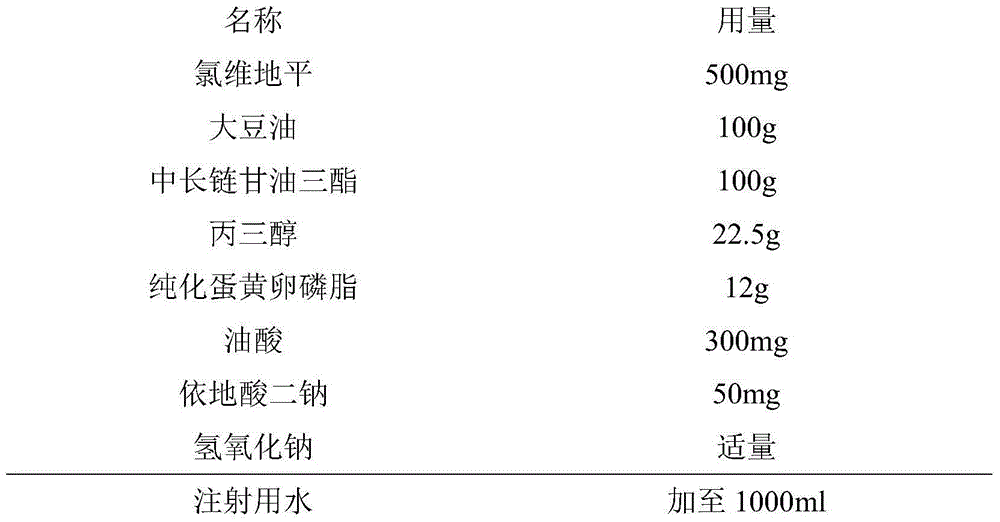
[0028]
[0029] Preparation Process:
[0030] (1) Preparation of the oil phase: under the protection of nitrogen, weigh the prescription amount of soybean oil and medium and long-chain triglycerides, heat to 60°C, add the prescription amount of clevidipine into the oil for injection, and stir at high speed to make it Dissolved as the oil phase; keep warm at about 70°C.
[0031] (2) Preparation of the water phase: Dissolve the prescription amount of edetate disodium and glycerol in water, adjust the pH to 8 with 1N sodium hydroxide solution, add lecithin and oleic acid to stir and disperse evenly, heat the water phase and keep it warm At around 70°C.
[0032] (3) Under high-speed shearing and stirring, mix the oil phase and the water phase at a ratio of 1:3 to 1:4, shear and stir to make colostrum, add water for injection to the prescribed amount, and shear at high speed for 1 to 2 hours.
[0033] (4) Homogenization: Homogenize the colostrum under high pressure at 500-800 ...
Embodiment 2
[0042]
[0043]
[0044] Preparation Process:
[0045] (1) Preparation of the oil phase: under the protection of nitrogen, weigh the prescribed amount of soybean oil, heat it to 60°C, add the prescribed amount of clevidipine into the oil for injection, and dissolve it with high-speed shearing and stirring, and use it as the oil phase; 70 Keep warm at about ℃.
[0046] (2) Preparation of the water phase: Dissolve the prescription amount of disodium edetate and glycerol in water, adjust the pH to 9 with 1N sodium hydroxide solution, add lecithin and sodium oleate to stir and disperse evenly, heat the water phase and Keep warm at around 70°C.
[0047] (3) Under high-speed shearing and stirring, mix the oil phase and the water phase at a ratio of 1:3 to 1:4, shear and stir to make colostrum, add water for injection to the prescribed amount, and shear at high speed for 1 to 2 hours.
[0048] (4) Homogenization: Homogenize the colostrum under high pressure at 500-800bar to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com