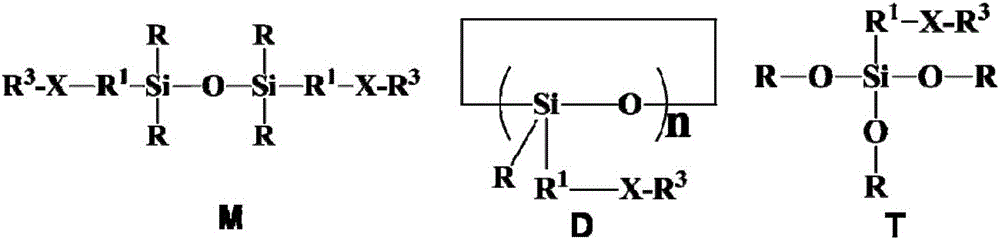
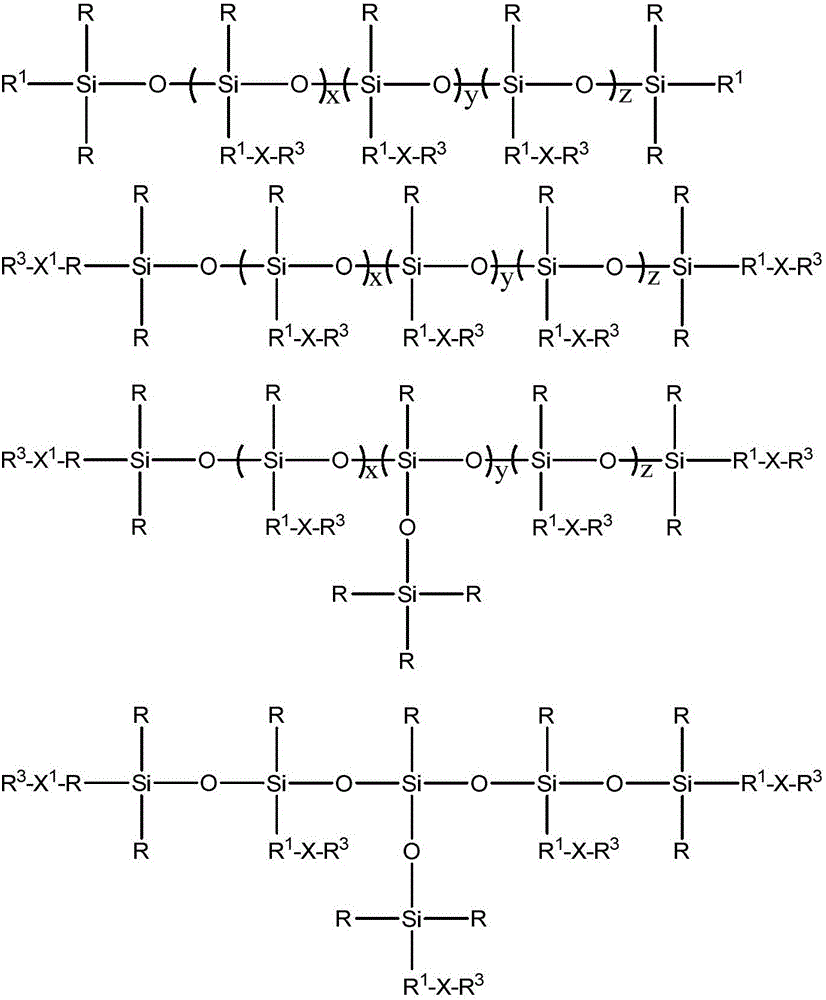
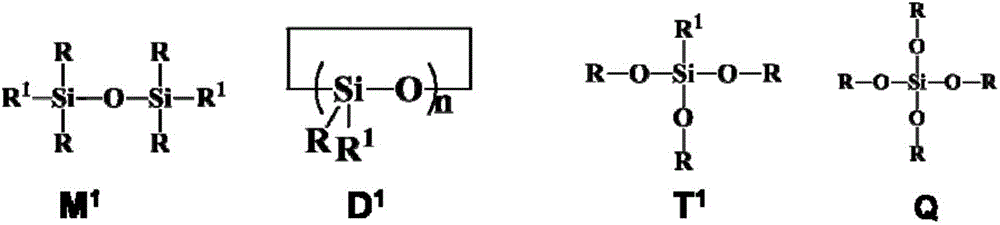Fluorine-containing alkenyl polysiloxane, preparation method thereof and application
A technology of alkenyl polysiloxane and alkenyl siloxane, which can be used in coatings and other directions, can solve problems such as increasing environmental costs and potential safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 100g tetravinyltetramethylcyclotetrasiloxane (344.66) (Aladdin), 278.64g heptadecafluoro-1-decanethiol (Aladdin) (480.18) and 0.5g 1,5-diazabicyclo [4.3.0] Non-5-ene (DBN) (Aladdin) catalyst was dissolved in 1000 mL of butanone, mixed evenly, and reacted at 20°C for 20 min. pass 1 The reaction was monitored by H-NMR. The unreacted monomer and solvent were removed by rotary evaporation under reduced pressure to obtain 360 g of light yellow oily liquid with a yield of 95.1%.
[0065] React 360g of the fluorine-containing segment compound obtained above, 300g of octamethylcyclotetrasiloxane (Aladdin), and 3.3g of trifluoromethanesulfonic acid at 0°C for 15 minutes, and then add 71g of hexamethyldisiloxane Alkane (162.38) (Aladdin) was capped for 0.5h. Finally, 2 g of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) (Aladdin) base was added for neutralization and filtration to obtain 673 g of oily liquid I with a yield of 92.1%.
Embodiment 2
[0067] 100g tetravinyltetramethylcyclotetrasiloxane (344.66) (Aladdin), 278.64g heptadecafluoro-1-decanethiol (Aladdin) (480.18) and 0.5g 1,5-diazabicyclo [4.3.0] Dissolve non-5-ene (DBN) catalyst in 1000ml of butanone, mix well, and react at 20°C for 20min. pass 1 The reaction was monitored by H-NMR. The unreacted monomer and solvent were removed by rotary evaporation under reduced pressure to obtain 360 g of light yellow oily liquid with a yield of 95.1%.
[0068] The above obtained 360g fluorine-containing segment compound, 300g octamethylcyclotetrasiloxane, and 3.3g trifluoromethanesulfonic acid were reacted at 0°C for 15min, and then 149g divinyltetramethylsiloxane ( 186.4) (Aladdin) was capped for 0.5h. Finally, 2 g of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) was added for neutralization and filtration to obtain 728 g of oily liquid II with a yield of 90.0%.
Embodiment 3
[0070] 100g of tetravinyltetramethylcyclotetrasiloxane (344.66), 278.64g of heptadecafluoromercaptan (480.18) and 0.5g of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN ) catalyst was dissolved in 1000 mL of methyl ethyl ketone, mixed evenly, and reacted at 20° C. for 20 min. pass 1 The reaction was monitored by H-NMR. The unreacted monomer and solvent were removed by rotary evaporation under reduced pressure to obtain 360 g of light yellow oily liquid with a yield of 95.1%.
[0071]React 360g of the above-obtained fluorine-containing segment compound and 3.3g of trifluoromethanesulfonic acid at 0°C for 15min, then add 149g of divinyltetramethylsiloxane (186.4) (Aladdin) for end-capping treatment 0.5h. Finally, 2 g of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) (Aladdin) was added for neutralization and filtration to obtain 460 g of oily liquid III with a yield of 90.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com