One group of DNA (Deoxyribose Nucleic Acid) molecules, recombinant vector, recombinant ketogulonigenium sp and method for producing 2-keto-L-gulonic acid
A ketoglobulina and DNA molecule technology, applied in the field of genetic engineering, can solve the problems of difficult and incomplete molecular operations, and achieve the effects of promoting growth, increasing acid production level and acid production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] In the present invention, after the preparation of the recombinant vector is completed, it is transferred into Bacillus ketoglobulina by electroporation under the condition of 2000V, and the specific method is as follows:
[0048] 1. Preparation of K.vulgare (Ketoglobulina) Competent Cells
[0049] K.vulgare glycerol was activated on a solid plate, transferred to 50mL HJ medium, and cultured for 13h until the bacteria reached the late exponential phase; put 40mL of bacteria in a 50mL pre-cooled centrifuge tube, and put it in an ice bath for 15min; freeze at 4°C Centrifuge at 4500rpm for 15min in a centrifuge, discard the supernatant; wash with pre-cooled 10% glycerol, resuspend the cells, centrifuge at 4500rpm for 15min, and remove the supernatant; repeat the previous step; add 30mL of sterilized pre-cooled Cells were resuspended in ultrapure water, centrifuged at 4500rpm for 15min, and the supernatant was discarded; 900μL of pre-cooled 10% glycerol was added to resuspe...
Embodiment 1
[0055] Embodiment 1: the preparation of recombinant ketoglobulina of the present invention
[0056] 1. In vitro amplification of the recombinant vector
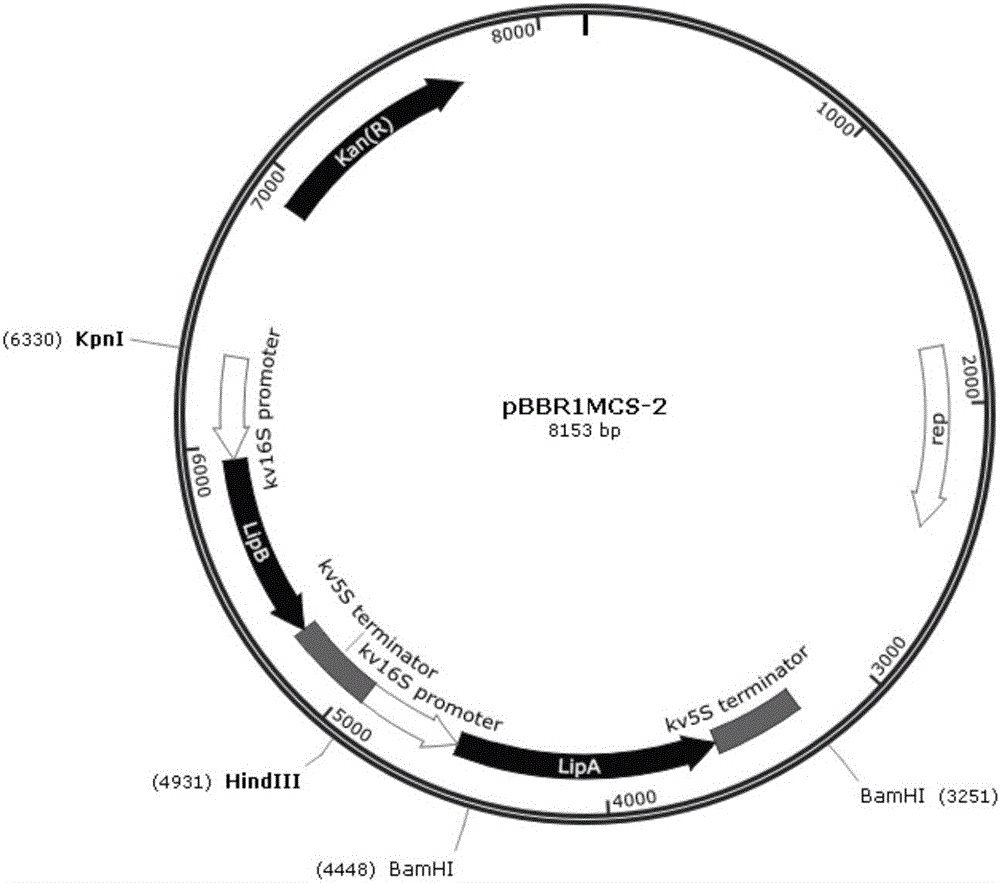
[0057] According to the preparation scheme of the DNA molecules and recombinant vectors provided by the present invention, they are synthesized by biotechnology companies, and the vector plasmid map after recombination is shown in figure 1 , wherein the embedded sequence is as shown in SEQ ID NO:3 sequence, that is, KpnI restriction site-16S promoter-SEQ ID NO: sequence shown in 1-5S terminator-HindIII restriction site-6S promoter-SEQ The sequence shown in ID NO: 2-5S terminator-BamHI restriction site.
[0058] The recombinant vector was transformed through the classical heat shock method commonly used in large intestine, and the competent cells used were purchased from Beijing Bomed Technology Development Co., Ltd. BDH5α. After transformation, an appropriate LB resistance plate was selected according to the resistance carr...
Embodiment 2
[0074] Embodiment 2: the shaking flask fermentation test of recombinant ketoglobulina of the present invention
[0075] 1. Test groups
[0076] kv: primitive ketogluconic acid bacteria;
[0077] kv-lxs: the original ketogalactobacillus added with exogenous lipoic acid;
[0078]lip-kv: Recombinant Bacteria ketoclonicus according to the present invention;
[0079] lip-kv-lxs: the recombinant ketoglobulina of the present invention added with exogenous lipoic acid;
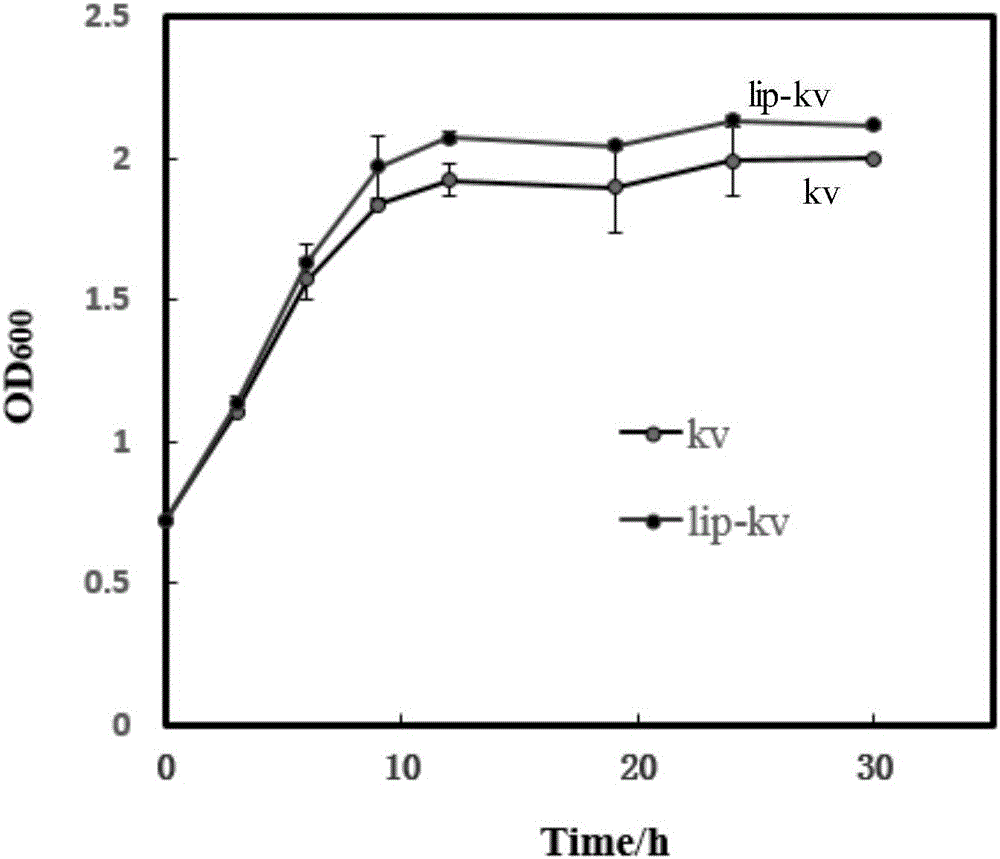
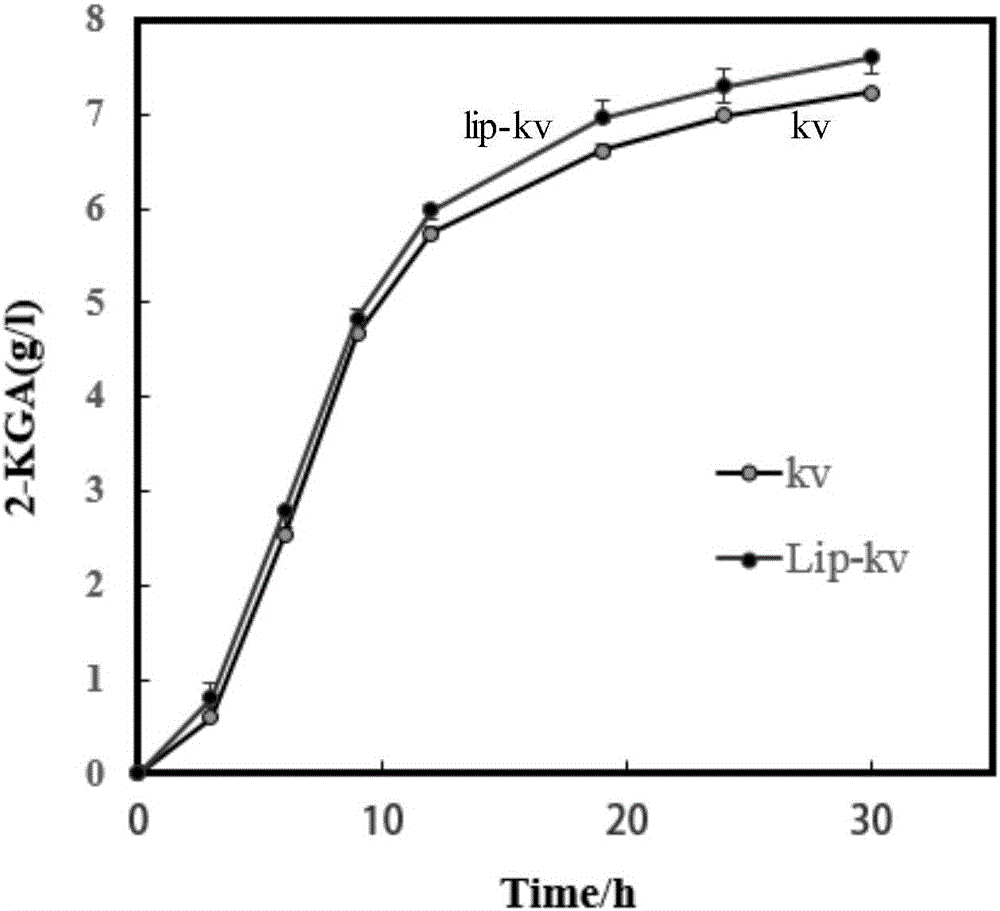
[0080] 2. Contrastive test of shake flask fermentation at different time points between kv group and lip-kv group
[0081] The strains of each group were first added to the solid seed medium and cultured at 30°C for 24h for activation, then transferred to the seed medium at the same initial OD and fermented in shake flasks at 30°C and 250r / min, 0h, 3h , 6h, 9h, 12h, 19h, 24h, 30h and other different time points for sampling and analysis.
[0082] Described solid seed culture medium is the seed culture medium afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com