Preparation method of slow-released microgranules, prepared slow-released microgranules and application thereof
A technology for slow-release microparticles and microparticles, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve the problems of unstable release, waste, disadvantage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
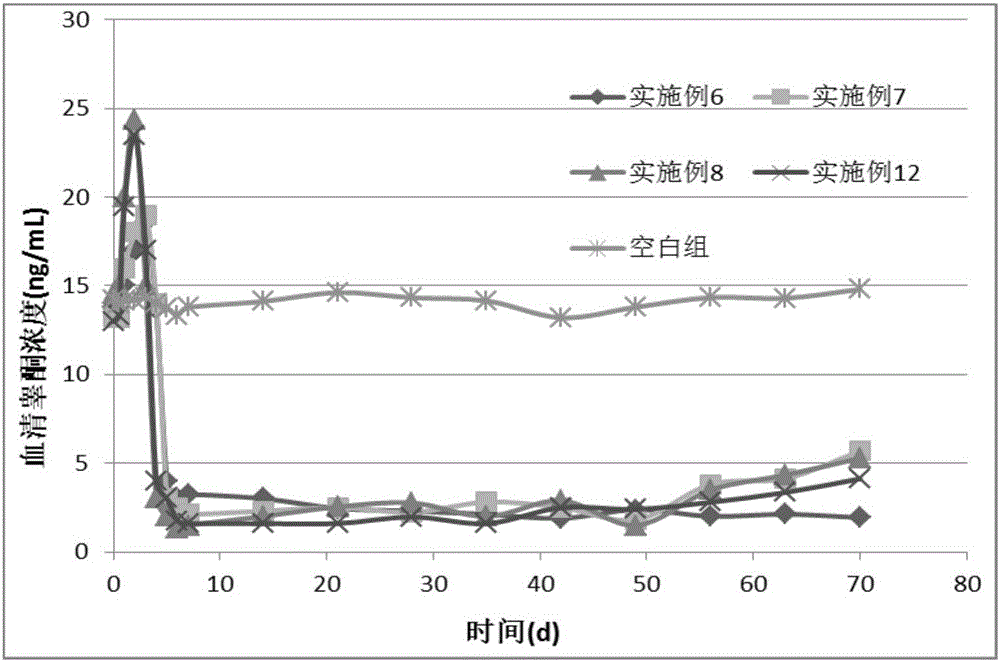
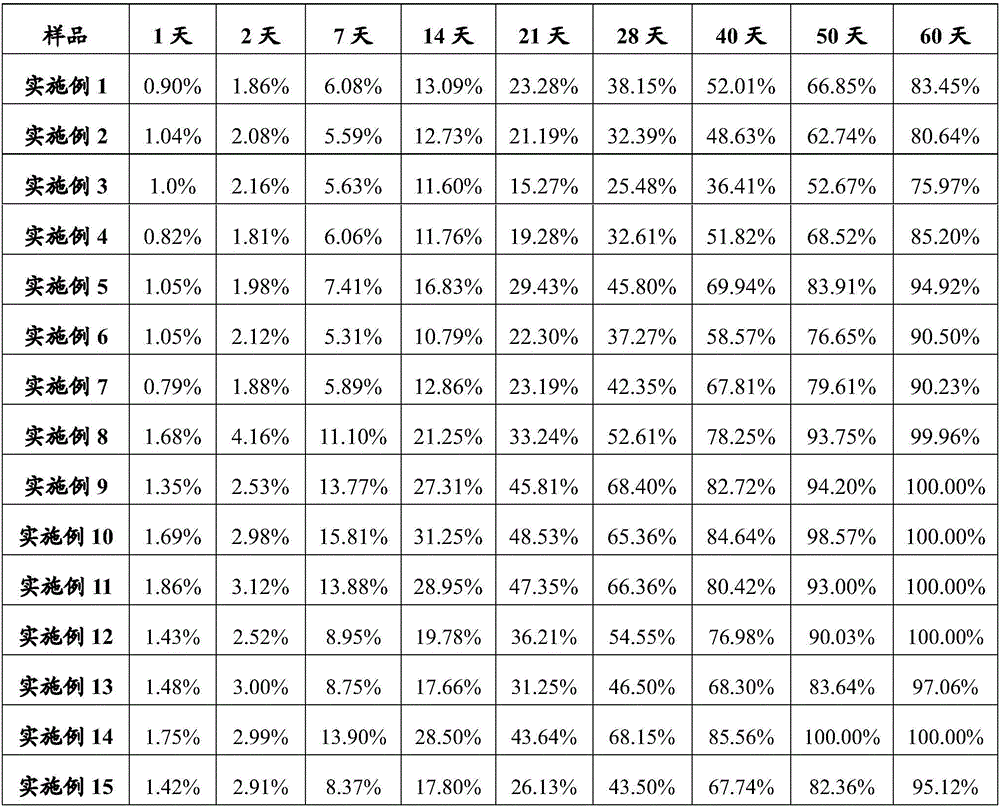
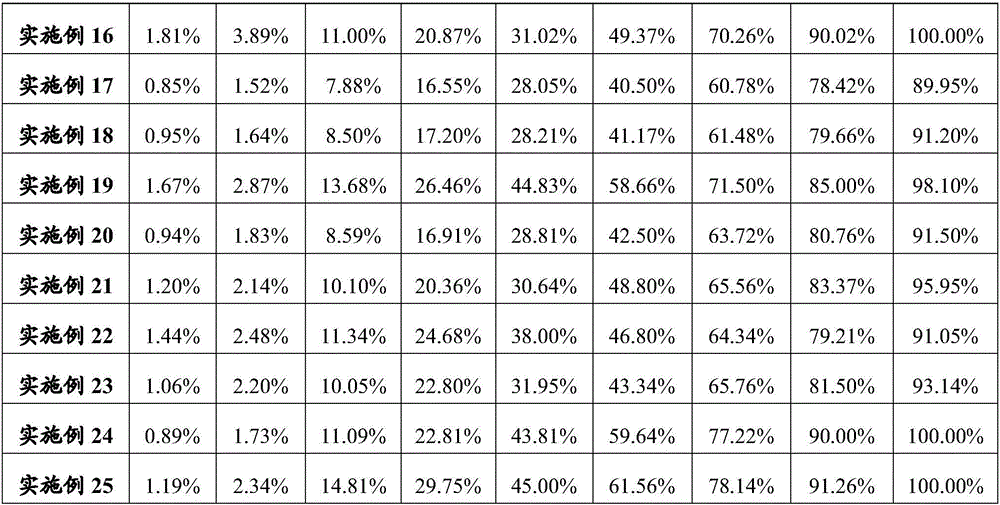
Embodiment 1
[0103] Example 1 Preparation of Glucagon / PLA Microparticles
[0104] (1) Preparation of solid dispersion
[0105] Dissolve 0.99g PLA (molecular weight 25kDa, terminal ester group) in about 5.50mL glacial acetic acid, then add 0.01g glucagon acetate, 0.05g xylitol and 0.05g zinc hydroxide, vortex to dissolve, then slowly Inject into stirred anhydrous diethyl ether (8°C) to produce a white precipitate, collect the white precipitate and extract about 5 times with anhydrous diethyl ether, dry the precipitate in a vacuum oven for 24 hours (10°C) to obtain a solid Dispersions.
[0106] (II) Preparation of microparticles
[0107] The solid dispersion obtained in step I was uniformly dispersed in about 5.50 g of dichloromethane to obtain an internal oil phase, and then the internal oil phase was injected into 200 mL of 0.05% (w / w) lecithin / peanut oil that had been pre-heated to about 10°C solution, and emulsified using a turbine homomixer to prepare S / O / O emulsion (running speed ...
Embodiment 2
[0108] Example 2 Preparation of Ziconotide / PLGA Microparticles
[0109] (1) Preparation of solid dispersion
[0110] Dissolve 0.97g PLGA (molecular weight 25KDa, monomer ratio 90 / 10, terminal ester group) in about 5.39mL acetonitrile, then add 0.03g ziconotide acetate, 0.05g xylitol and 0.03g zinc chloride, vortex Dissolved under low temperature, and then slowly injected into cyclohexane (6°C) under stirring to produce a white precipitate, which was collected and extracted about 5 times with cyclohexane, and dried in a vacuum oven for 24 hours after the precipitate was collected ( 10°C) to obtain a solid dispersion.
[0111] (II) Preparation of microparticles
[0112] The solid dispersion obtained in step I was uniformly dispersed in about 5.39 g of chloroform to obtain an internal oil phase, and then the internal oil phase was injected into 290 mL of a 0.1% (w / w) lecithin / liquid paraffin solution that had been pre-heated to about 8°C , and use a high-speed homogenizer to p...
Embodiment 3
[0113] Example 3 Preparation of Tecoctide / PLGA Microparticles
[0114] (1) Preparation of solid dispersion
[0115] Dissolve 0.95g PLGA (molecular weight 30kDa, monomer ratio 85 / 15, carboxyl-terminated) in a mixture of about 6.33mL glacial acetic acid and acetonitrile, then add 0.05g tecosetide acetate, vortex to dissolve, and then slowly Inject into stirred n-hexane (6°C) to produce a white precipitate. Collect the white precipitate and extract it with n-hexane for about 5 times. After collecting the precipitate, dry it in a vacuum oven for 24 hours (10°C) to obtain a solid dispersion .
[0116] (II) Preparation of microparticles
[0117] The solid dispersion obtained in step I was uniformly dispersed in about 6.33g of tetrachlorethylene to obtain an internal oil phase, and then the internal oil phase was injected into 480mL of 0.25% (w / w) lecithin / corn which had been pre-heated to about 6°C Oil solution, and use SPG membrane emulsifier to prepare S / O / O emulsion (membran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com