Pharmaceutical composition for treating staphylococcus aureus infection
A technology for staphylococcus infection and staphylococcus, which is applied in the field of medicine, can solve problems such as inability of patients to be cured, and achieve the effects of low price, inhibitory activity, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
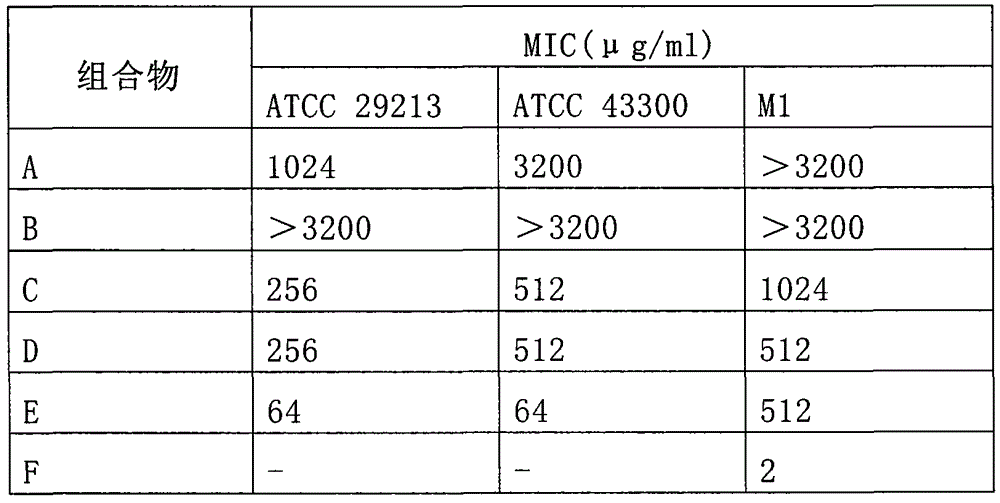
[0033] The pharmaceutical composition was formulated according to Table 1 below.
[0034] Table 1
[0035] combination
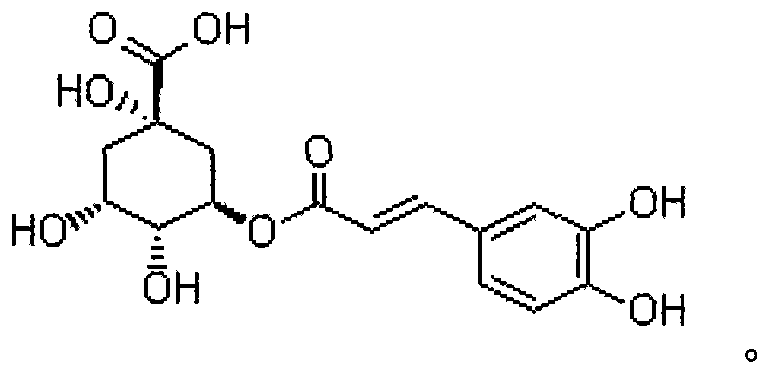
Chlorogenic acid (mg)
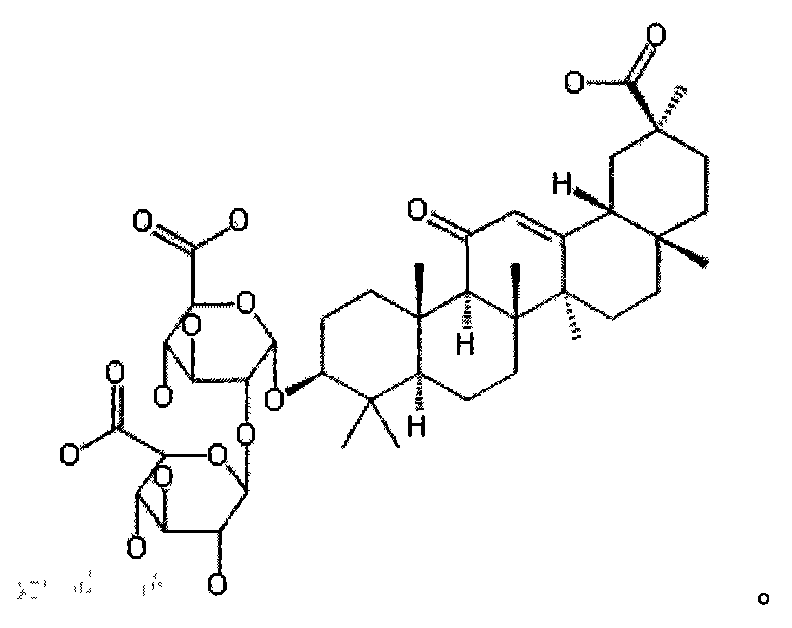
Glycyrrhizic acid (mg)
Erythromycin (mg)
Vancomycin (mg)
A
30
0
0
0
B
0
10
0
0
C
40
10
0
0
D
45
10
0
0
E
50
10
10
0
F
30
10
0
10
[0036] Determination of minimum inhibitory concentration (MIC):
[0037] Take the compositions of each group prepared according to Table 1 and carry out a series of multiple dilutions in the MH broth medium, and adjust the initial bacterial concentration to 1.0×10 6 CFU / ml. After incubating at 35°C for 24 hours, the lowest concentration of antibacterial drug without precipitation is its minimum inhibitory concentration (MIC). Erythromycin (ERY) and vancomycin (VAN) were used as controls.
[0038] The results of the in vitro antibacterial test are shown in Table 2.
[...
Embodiment 2
[0046] Balb / C mouse sepsis model: established according to the method of cecal ligation and puncture (CLP). For the establishment method, please refer to the literature J.Vis.Exp.(62), e3926, doi: 10.3791 / 3926 (2012). Animal survival time was calculated from the completion of surgery.
[0047] The composition of the present invention (Group C in Example 1) was dissolved in physiological saline, and administered by intraperitoneal injection according to the following doses. The first dose was given 24 hours after CLP, and then every 24 hours for a total of 3 doses. Divided into 3 experimental groups, the doses of each group were 2.0, 4.0, and 8.0 mg / kg body weight respectively.
[0048] The control group was only injected with the same volume of normal saline.
[0049] The results showed that 2.0, 4.0, 8.0 mg / kg body weight compositions increased the eighth day survival rate of septic animals from 21% to 50%, 57%, 85%, respectively. These results indicate that the compositio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com