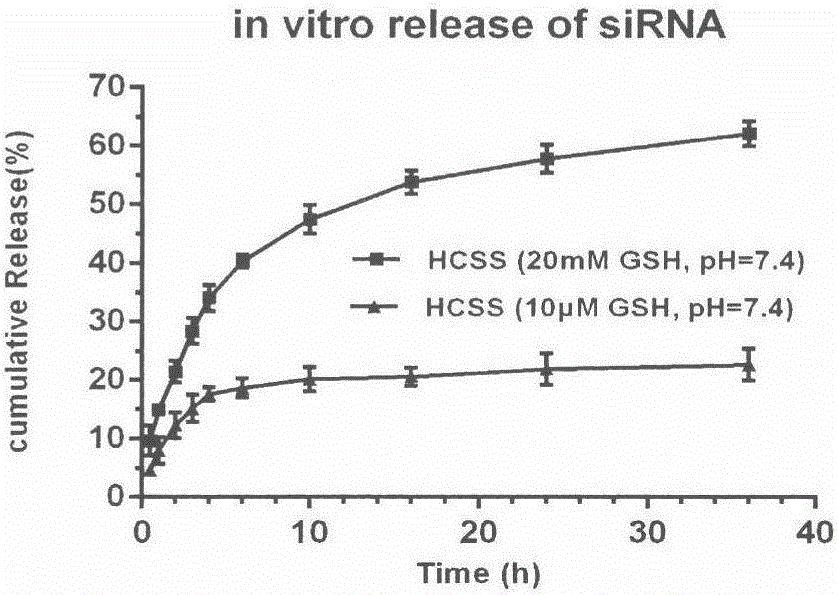
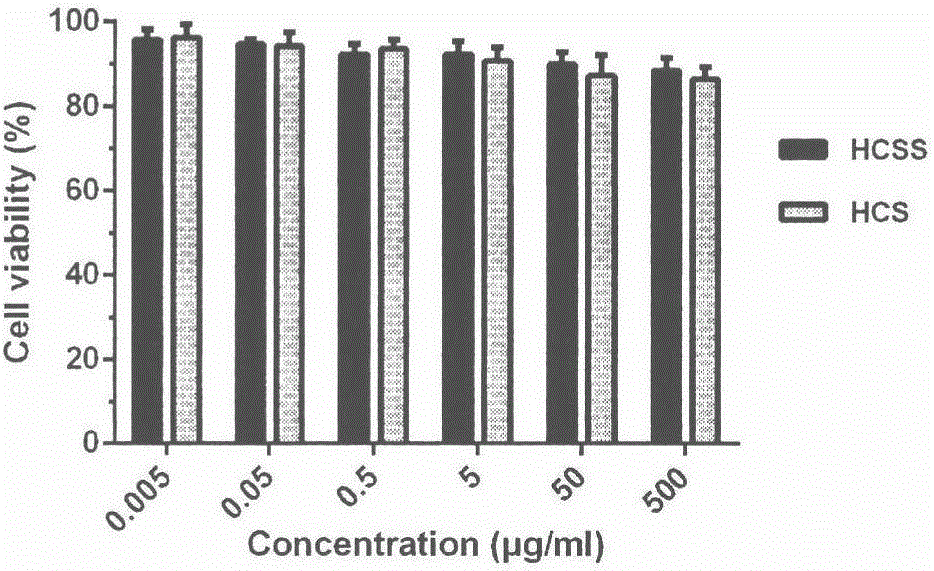
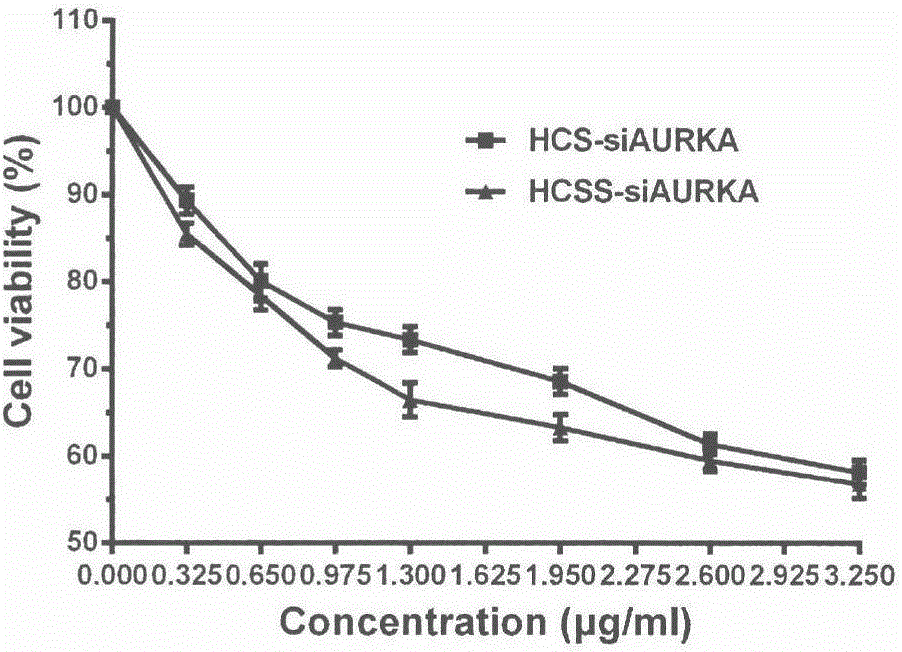
Application of chitosan reduction-sensitive system as gene drug delivery carrier
A technology of gene carrier and chitosan derivative, which is applied in the field of preparation of the carrier, achieves the effects of high safety, low toxicity and side effects, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of hydroxyethyl chitosan-histidine reduction sensitive copolymer
[0047] Dissolve 1g of chitosan in isopropanol solution, add 10ml of 50% NaOH solution, after alkalization, add 10ml of ethylene oxide to the reaction solution, and stir magnetically for 2-24 hours under the condition of 35-50°C oil bath, Adjust the pH of the reaction solution to 7, dialyze and freeze-dry to obtain water-soluble hydroxyethyl chitosan.
[0048] 0.3g hydroxyethyl chitosan is dissolved in the mixed solvent of water and methanol (v / v=1:1), add 0.6g S-aminoethyl-3,4-dithiopropionic acid, 0.547g EDC React with 0.328g NHS for 12h, remove methanol by rotary evaporation, dialyze (MWCO=14000) for 2-3 days, and lyophilize to obtain a chitosan intermediate whose free segment is an amino group.
[0049] React 0.25g of chitosan intermediate, 0.22g of histidine, 1.36g of EDC and 0.818g of NHS for 12 hours. After the reaction, use excess acetone to precipitate the polysacch...
Embodiment 2
[0050] Embodiment 2: the preparation of hydroxyethyl chitosan-spermine reduction sensitive copolymer
[0051] Dissolve 1g of chitosan in acetic acid solution, add 10ml of 50% NaOH solution, after alkalization, add 10ml of ethylene oxide to the reaction solution, stir magnetically for 2-24 hours under the condition of 35-50°C oil bath, and put the reaction The pH of the solution was adjusted to 7, and the water-soluble hydroxyethyl chitosan was obtained by dialysis and freeze-drying.
[0052] 0.3g hydroxyethyl chitosan is dissolved in the mixed solvent of water and methanol (v / v=1: 1), add 0.6g3,3'-dithiodipropionic acid, 0.547g EDC and 0.328g NHS, React for 12 hours, remove methanol by rotary evaporation, dialyze (MWCO=14000) for 2-3 days, and lyophilize to obtain a chitosan intermediate with a carboxyl group as the free segment.
[0053] 0.3g chitosan intermediate, 0.1337g spermine, 0.1785g EDC and 0.1065g NHS were reacted for 24 hours, dialyzed (MW=14000) for 3 days, and fr...
Embodiment 3
[0054] Embodiment 3: the preparation of hydroxyethyl chitosan-arginine reduction sensitive copolymer
[0055] Dissolve 1g of chitosan in acetic acid solution, add 10ml of 50% NaOH solution, after alkalization is completed, add 10ml of ethylene oxide to the reaction solution, stir magnetically for 2-24h under the condition of 35-50°C oil bath, and put the reaction The pH of the solution was adjusted to 7, and the water-soluble hydroxyethyl chitosan was obtained by dialysis and freeze-drying.
[0056] 0.3g hydroxyethyl chitosan is dissolved in the mixed solvent of water and methanol (v / v=1:1), add 0.6g S-aminoethyl-3,4-dithiopropionic acid, 0.547g EDC React with 0.328g NHS for 12h, remove methanol by rotary evaporation, dialyze (MWCO=14000) for 2-3 days, and lyophilize to obtain a chitosan intermediate whose free segment is an amino group.
[0057] 0.3g chitosan intermediate, 0.115g arginine, 0.154g EDC and 0.092g NHS were reacted for 12h, dialyzed (MW=14000) for 3 days, and fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com