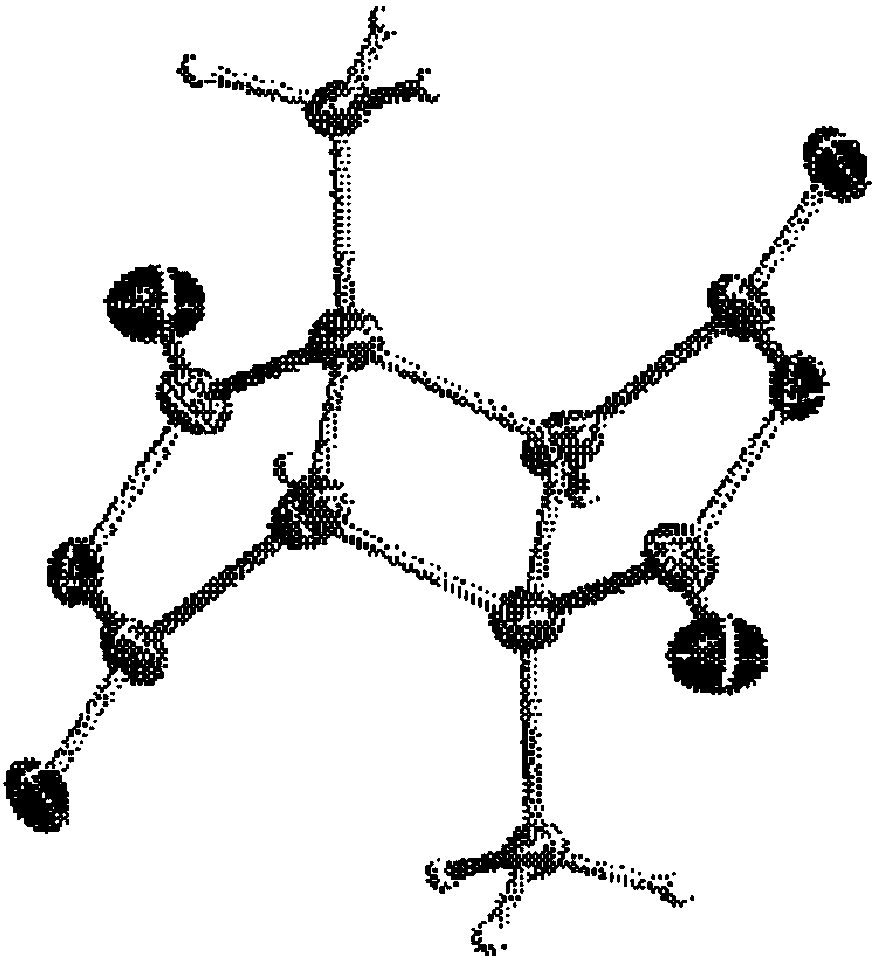
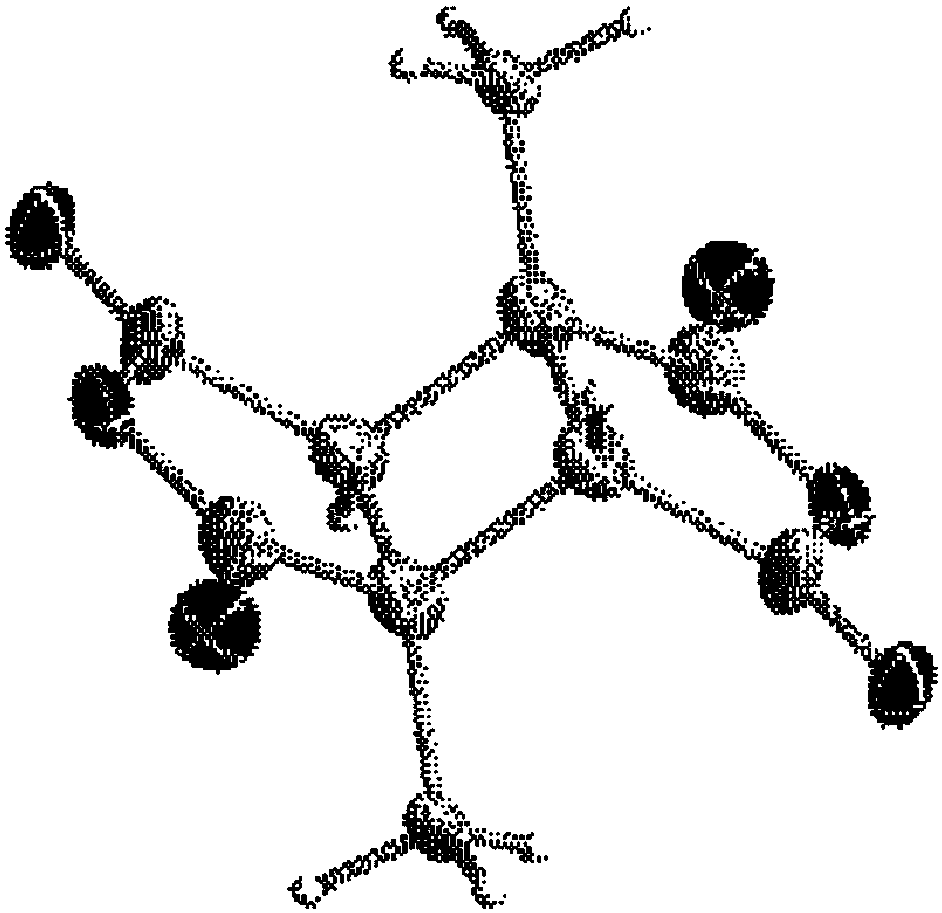
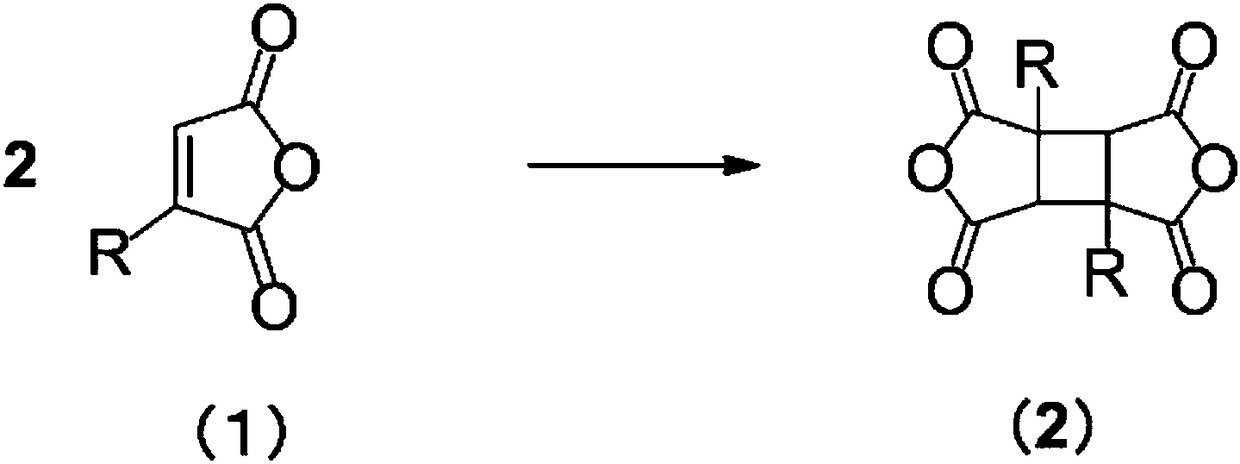
Process for producing cyclobutane tetracarboxylic acid derivatives
A technology of cyclobutane tetracarboxylic acid and its manufacturing method, which is applied in organic chemistry and other fields, can solve problems such as insufficient photoreaction efficiency, and achieve high photoreaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0073] Examples are listed below to explain the present invention in more detail, but the present invention is not limited to these examples. It should be noted that the analysis methods used in the examples are as follows.
[0074]
[0075] Device: GC-2010Plus (manufactured by Shimadzu Corporation),
[0076] Column: DB-1 (manufactured by Agilent Technologies, Ltd.) 0.25 mm in diameter × 30 m in length, 0.25 μm in film thickness,
[0077] Carrier gas: He, detector: FID, sample injection volume: 1μL, injection port temperature: 160°C, detector temperature: 220°C, column temperature: 70°C (20min)-40°C / min-220°C (15min) , Split ratio: 1:50, internal standard substance: butyl lactate.
[0078] 1 H NMR analysis conditions>
[0079] Device: Fourier transform type superconducting nuclear magnetic resonance device (FT-NMR) INOVA-400 (manufactured by Varian) 400MHz,
[0080] Solvent: DMSO-d6, internal standard substance: tetramethylsilane (TMS).
[0081]
[0082] Device: DSC1 (manufactured by Me...
Embodiment 10
[0101]
[0102] In a nitrogen atmosphere, in a 300mlLPyrex (registered trademark) glass five-necked flask was put 3.5g (31.2mmol) of citraconic anhydride (CA), 0.70g (3.23mmol) of 4-chlorobenzophenone (ClBP), Conic anhydride (CA) (10 mol%) and 136.5 g (1515 mmol, 39.0 wt times of citraconic anhydride (CA)) and dimethyl carbonate were stirred and dissolved with a magnetic stirrer.
[0103] Then, while stirring at 10-15°C, a 100W high-pressure mercury lamp was irradiated for 1 hour. As a result of the quantitative analysis of the reaction liquid by gas chromatography after irradiation, the residual rate of citraconic anhydride (CA) was 69.1%. In addition, 0.2 g of the reaction liquid in the reactor was taken, and the solvent was distilled off at 70-80 Torr with an evaporator. by 1 H NMR analysis confirmed that the obtained crude product was a mixture containing 1,3-DM-CBDA and 1,2-DM-CBDA (1,3-DM-CBDA:1,2-DM-CBDA=44.6:55.4).
Embodiment 11~13
[0105] A series of operations were performed in the same manner as in Example 10, and it was implemented so that the type of sensitizer became the value shown in the following table. In addition, the residual rate of citraconic anhydride, the reaction rate, and the production ratio of 1,3-DM-CBDA to 1,2-DM-CBDA in the reaction solution obtained here were calculated, and the results are shown together with the results obtained in Example 10. In the table. It should be noted that the reaction rate in the table is calculated from the number of moles of citraconic acid used and the residual rate of citraconic acid at the time of the reaction for 1 hour.
[0106] [Table 2]
[0107]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com