Polysiloxane-carbamate elastomer containing Diels-Alder bond and preparation method of polysiloxane-carbamate elastomer
A technology of urethane and polysiloxane, applied in the field of materials, can solve the problem of poor mechanical properties of reversible covalently cross-linked polysiloxane, high reprocessing temperature, mechanical properties and self-healing properties cannot be improved at the same time, etc. problems, to achieve excellent water resistance and air permeability, good biocompatibility, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
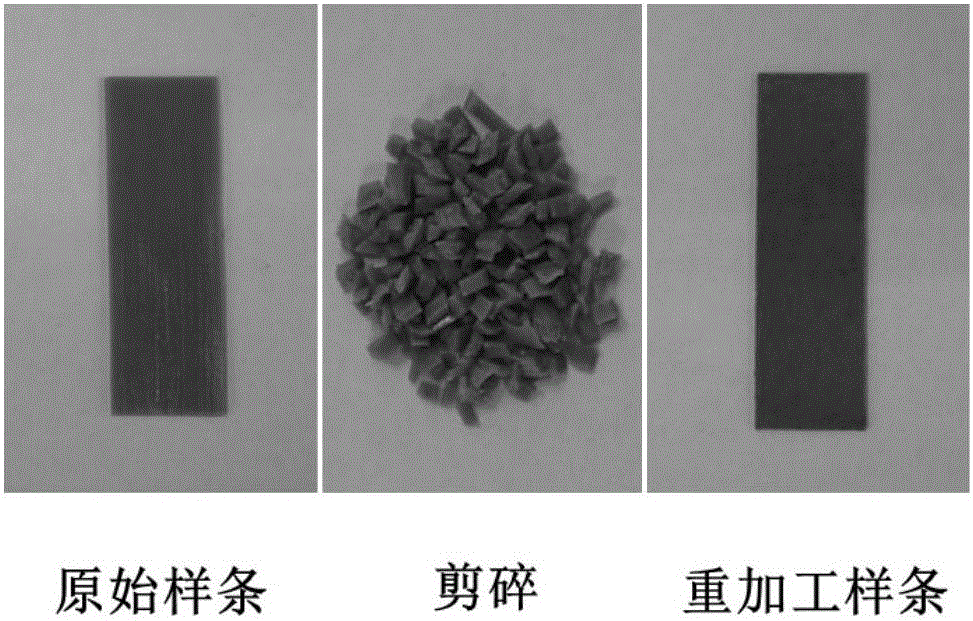
Examples
Embodiment 1
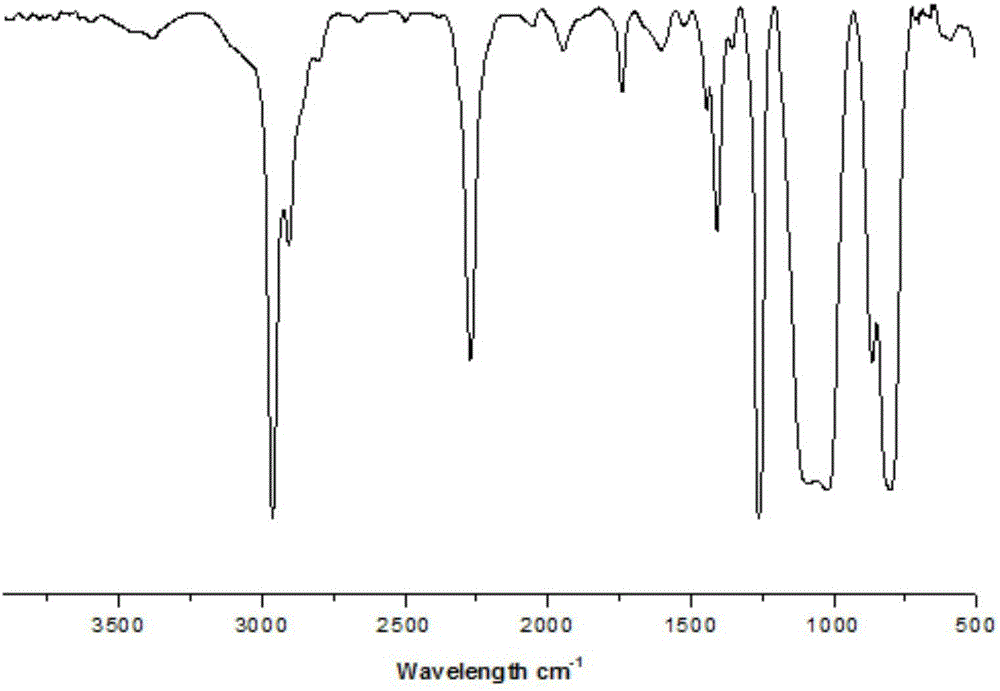
[0044] Synthesis of isocyanate-terminated polydimethylsiloxane: 10 g aminopropyl-terminated polydimethylsiloxane DMS-A12 (10 mmol), 4.5 g triethylamine (45 mmol) and 4.45 g triphosgene (15 mmol ) was dissolved in 300mL benzene, stirred and reacted at room temperature under nitrogen protection for 6 hours. After the reaction was completed, a brown liquid was obtained by filtration, and the solvent was removed by rotary evaporation at 60° C. to obtain ~9.9 g of a brown sticky substance. Its infrared spectrum is as figure 1 shown.
Embodiment 2
[0046] Synthesis of isocyanate-terminated polyε-caprolactone: Weigh polyε-caprolactone diol and diphenylmethane diisocyanate with the ratio of the amount of substances -OH / -NCO=2 / 1, under nitrogen protection Stir and react at 80°C for 2 hours to obtain a white solid isocyanate-capped polyε-caprolactone, which is sealed for future use.
Embodiment 3
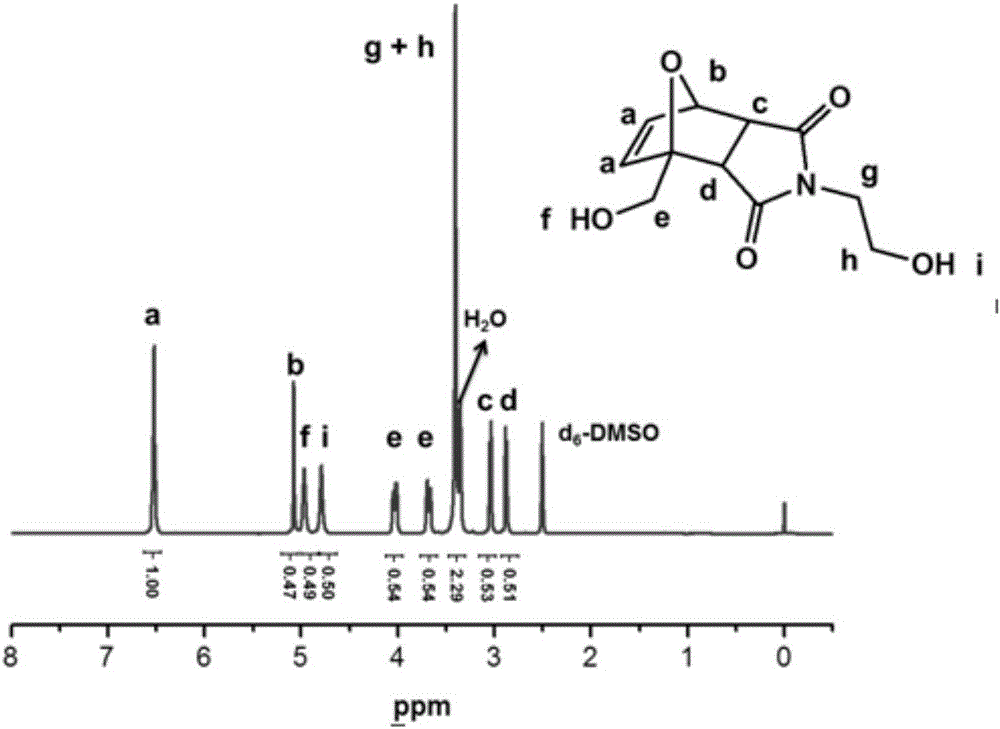
[0048] Synthesis of diols containing Diels-Alder bonds: Dissolve furan 1 and maleic anhydride 2 in an equivalent amount in excess 1,4-dioxane, stir and react at room temperature for 24 hours, mash the obtained precipitate, After suction filtration, ether washing, and drying, the product 3 was obtained; the equivalent amount of product 3 and ethanolamine were dissolved in methanol under ice bath conditions, stirred and reacted at 75°C for 24 hours, and the reaction mixture was cooled and crystallized in a refrigerator, and then After suction filtration, ether washing, and drying, the product 4 was obtained; the product 4 was added to excess toluene, stirred and refluxed at 130°C for 12 hours, then the solution was filtered, and the filtrate was cooled and crystallized in a refrigerator. washing and drying to obtain the product 5; the equivalent amount of the product 5 and furfuryl alcohol were stirred and reacted in toluene at 80°C for 24 hours, and the obtained precipitate was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com