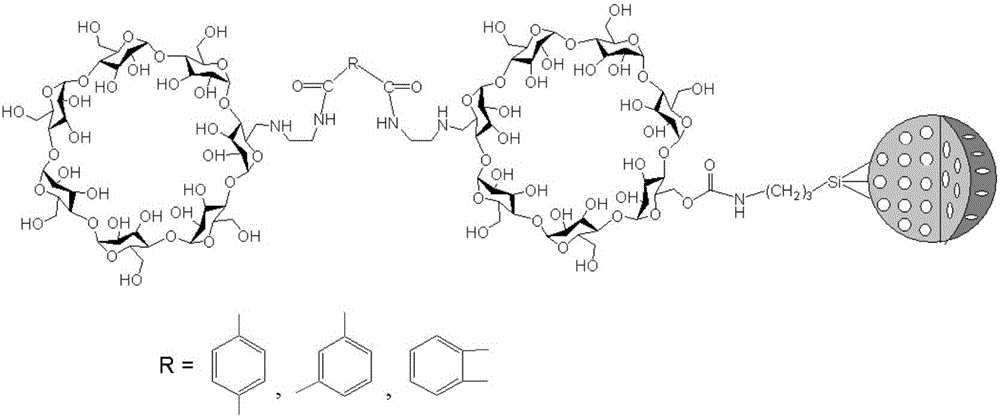
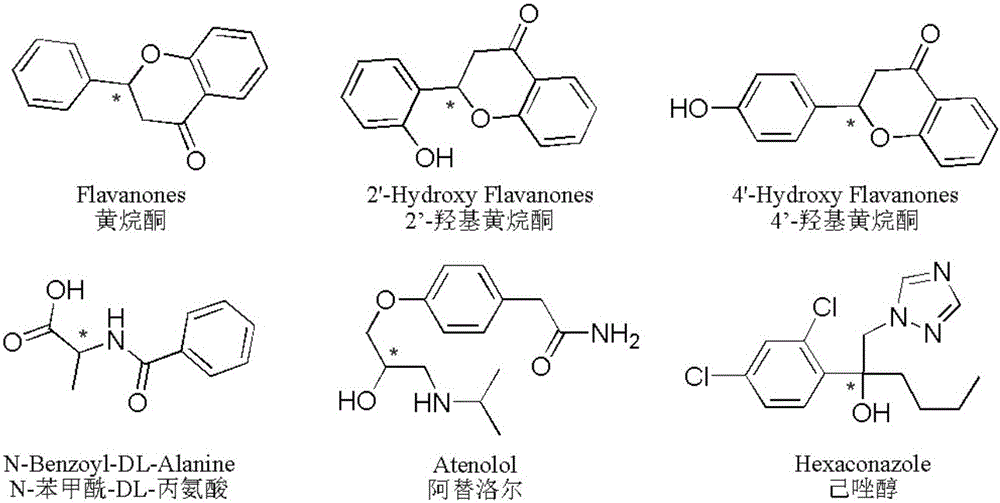
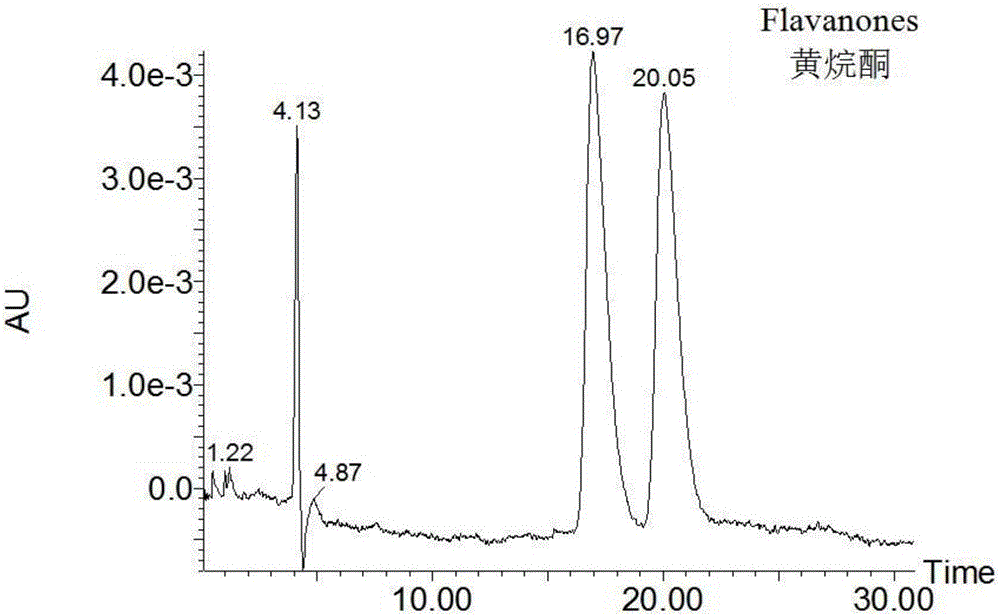
Phenylene ethylenediamine derivatized beta-cyclodextrin bonded silica gel and application thereof
A phthaloyl ethylene diamine and phthaloyl ethylene diamine-based technology is applied in the field of preparation of bonded SBA-15 silica gel chiral stationary phase, and can solve the problem of poor chiral separation performance of chiral stationary phase materials and other problems, to achieve the effect of improving the chiral separation ability and range, low preparation cost and high bonding amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (1) According to the ratio of phthalic acid (mmol): thionyl chloride (ml) of 1.0:2.0, add phthalyl and thionyl chloride into a round bottom flask, stir and place in a 75°C oil bath Heated to reflux for 1.5h. Then the unreacted thionyl chloride was evaporated under reduced pressure to obtain phthaloyl chloride;
[0066] (2) Under nitrogen atmosphere, according to 6-(ethylenediamino)-β-cyclodextrin (mmol): phthaloyl chloride (mmol): anhydrous N, N-dimethylformamide (ml) as 2.0~2.5:1.0:25~35 ratio, dissolve 6-(ethylenediamino)-β-cyclodextrin in anhydrous N,N-dimethylformamide, add phthaloyl chloride under ice bath , and then magnetically stirred at room temperature for 4h to 6h to obtain a solution containing 6-(phthaloylethylenediamine)-β-cyclodextrin. This step is not separated and purified, and the next step reaction is continued;
[0067] (3) Under nitrogen atmosphere, according to 6-(ethylenediamino)-β-cyclodextrin (mmol) in (2): 3-isocyanatopropyl trialkoxysilane ...
Embodiment 2
[0077] Take SBA-15 (400m 2 / g) Activated silica gel 2.5g as the base.
[0078] (1) According to the ratio of phthalic acid (mmol): thionyl chloride (ml) of 1.0:2.0, add phthalic acid and thionyl chloride into a round-bottomed flask, under stirring, in 75 ℃ oil The bath was heated to reflux for 1.5h. Then the unreacted thionyl chloride was evaporated under reduced pressure to obtain phthaloyl chloride;
[0079] (2) Under nitrogen atmosphere, according to 6-(ethylenediamino)-β-cyclodextrin (mmol): phthaloyl chloride (mmol): anhydrous N,N-dimethylformamide (ml) In the ratio of 2.0:1.0:25, dissolve 6-(ethylenediamino)-β-cyclodextrin in anhydrous N,N-dimethylformamide, add phthaloyl chloride under ice-cooling, room temperature The reaction was carried out under magnetic stirring for 4 hours to obtain a solution containing 6-(phthaloylethylenediamine)-β-cyclodextrin. This step is not separated and purified, and the next step reaction is continued;
[0080] (3) Under a nitrogen ...
Embodiment 3
[0087] Take SBA-15 (500m 2 / g) Activated silica gel 2.5g as the base.
[0088] (1) According to the ratio of phthalic acid (mmol): thionyl chloride (ml) of 1.0:2.0, add phthalyl and thionyl chloride into a round bottom flask, stir and heat at 75°C The bath was heated to reflux for 1.5h. Then the unreacted thionyl chloride was evaporated under reduced pressure to obtain phthaloyl chloride;
[0089] (2) Under nitrogen atmosphere, according to 6-(ethylenediamino)-β-cyclodextrin (mmol): phthaloyl chloride (mmol): anhydrous N,N-dimethylformamide (ml) In the ratio of 2.5:1.0:35, dissolve 6-(ethylenediamino)-β-cyclodextrin in anhydrous N,N-dimethylformamide, add phthaloyl chloride under ice-cooling, room temperature The reaction was carried out under magnetic stirring for 6 hours to obtain a solution containing 6-(phthaloylethylenediamine)-β-cyclodextrin. This step is not separated and purified, and the next step reaction is continued;
[0090] (3) Under a nitrogen atmosphere, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com