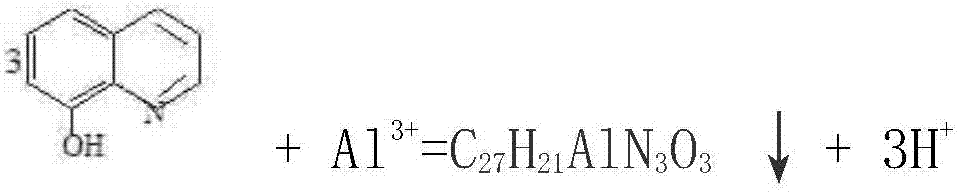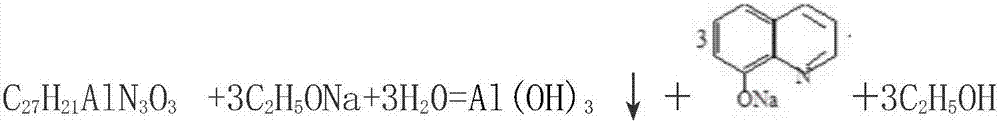A kind of separation method of aluminum in the production process of chromium salt
A technology of production process and separation method, applied in the preparation of chromate/dichromate, aluminum hydroxide, etc., can solve the problems of high water content, loss of chromium, small particle size, etc., and achieves easy filtration and low production cost. , the effect of small loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
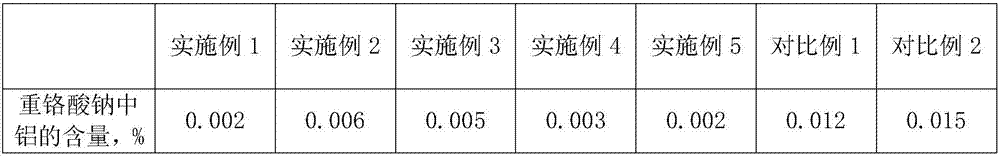
Examples
Embodiment 1
[0020] a, 100g of sodium dichromate alkaline solution is placed in the first container, and the sodium dichromate alkaline solution contains 0.75% trivalent aluminum ion by mass fraction, and then adds 20% sulfuric acid solution , stirring continuously to make the pH of the mixed solution=6.0;
[0021] B, constantly add the ethanol solution that mass fraction is 25% 8-hydroxyquinoline in the mixed solution, constantly stir, until there is no golden yellow precipitation to generate again, and reaction temperature is 65 ℃; The chemical reaction that takes place is as follows:
[0022]
[0023] C, filter the material obtained through the reaction of step b, the liquid obtained is a sodium dichromate solution, and the golden yellow precipitate of the gained is 8-hydroxyquinoline aluminum;
[0024] d. Place the golden-yellow precipitate of 8-hydroxyquinoline aluminum obtained in step c in a second container, and continuously add a 45% mass fraction of sodium ethoxide solution to...
Embodiment 2
[0028] a, 100g of sodium dichromate alkaline solution is placed in the first container, and the sodium dichromate alkaline solution contains trivalent aluminum ions with a mass fraction of 0.75%, then add a sulfuric acid solution with a mass fraction of 18% , stirring continuously to make the pH of the mixed solution=7.0;
[0029] B, continuously adding the ethanol solution of 30% 8-hydroxyquinoline in mass fraction to the mixed solution, stirring constantly, until no golden yellow precipitate is generated, and the reaction temperature is 70° C.;
[0030] C, filter the material obtained through the reaction of step b, the liquid obtained is a sodium dichromate solution, and the golden yellow precipitate of the gained is 8-hydroxyquinoline aluminum;
[0031] d. Place the golden-yellow precipitate of 8-hydroxyquinoline aluminum obtained in step c in a second container, and continuously add 50% sodium ethoxide solution to the second container until the 8-hydroxyquinoline aluminum...
Embodiment 3
[0034] a, 100g of sodium dichromate alkaline solution is placed in the first container, and the sodium dichromate alkaline solution contains trivalent aluminum ions with a mass fraction of 0.75%, and then a sulfuric acid solution with a mass fraction of 25% is added , stirring continuously to make the pH of the mixed solution=5.0;
[0035] b. Constantly adding an ethanol solution of 20% 8-hydroxyquinoline in a mass fraction to the mixed solution, stirring constantly, until no golden precipitate is generated, and the reaction temperature is 60° C.;
[0036] C, filter the material obtained through the reaction of step b, the liquid obtained is a sodium dichromate solution, and the golden yellow precipitate of the gained is 8-hydroxyquinoline aluminum;
[0037] d. Place the golden-yellow precipitate of 8-hydroxyquinoline aluminum obtained in step c in a second container, and continuously add a 40% mass fraction of sodium ethoxide solution to the second container until the 8-hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com