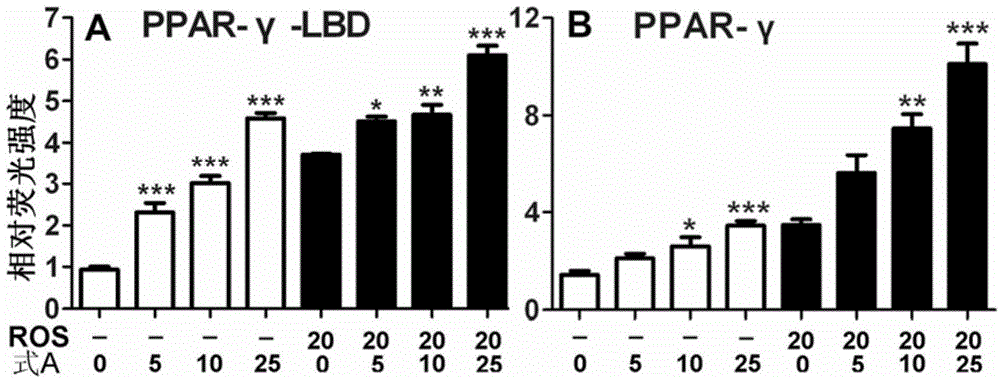
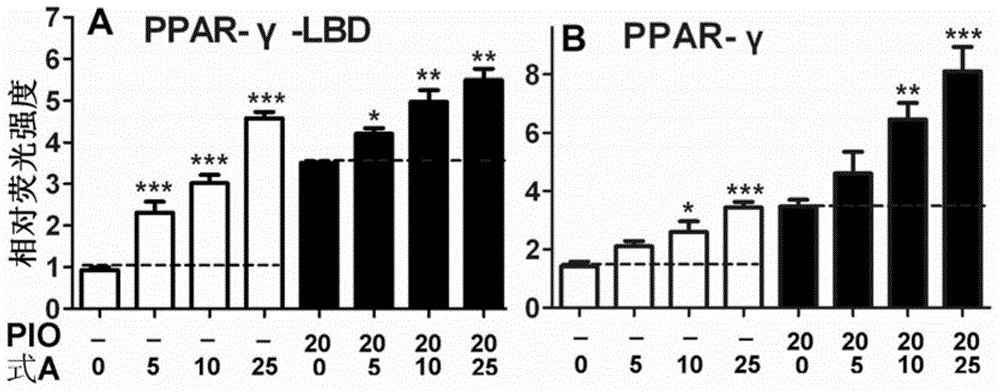
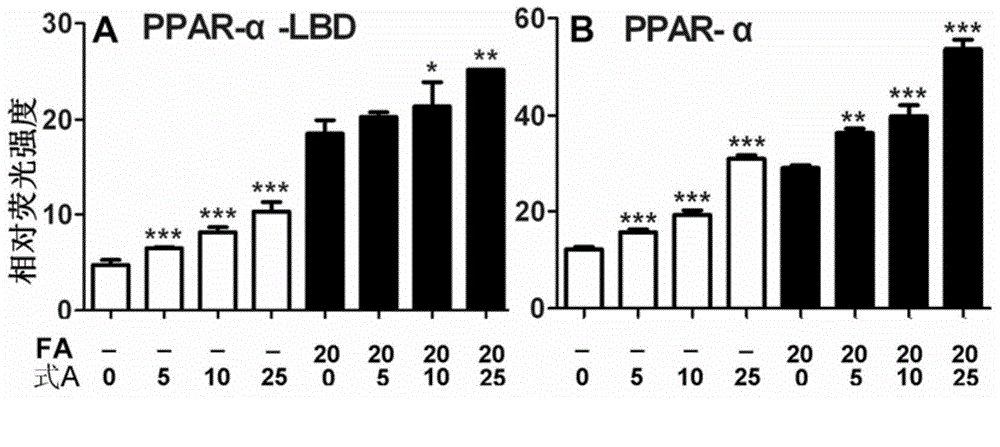
Application of bavachinin and analogs of bavachinin
A technology of bakuchiol dihydroflavonoid methyl ether and analogs, which can be applied in the field of medicine and can solve the problems of affecting and limiting clinical application, toxic and side effects, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of psoralen dihydroflavone methyl ether (compound of formula A)
[0040] 10.0kg of psoralen medicinal material was reflux extracted with 8 times the volume (80L) of 95% ethanol aqueous solution, refluxed for 2 hours each time, and refluxed for 3 times, the combined extracts were concentrated under reduced pressure to obtain the extract (approx. 800mL); add 1 times the amount (800mL) of water to the extract to suspend, then extract with petroleum ether (1000mL×3) and ethyl acetate (1000mL×3) successively, collect the ethyl acetate extract; recover under reduced pressure Carry out silica gel column chromatography after ethyl acetate, carry out gradient elution with petroleum ether and ethyl acetate successively (10:1-1:5); Use silica gel column chromatography of obtained fraction: use cyclohexane-acetone gradient first Elution (9:1-1:1), then use reverse column chromatography methanol-water gradient elution (60% methanol-80% methanol), and fi...
Embodiment 2
[0045] Embodiment 2: the preparation of formula B compound
[0046] Dissolve 20mg of psoralen dihydroflavone methyl ether [compound of formula A] in 1mL of pyridine, then add 1mL of acetic anhydride, let stand at room temperature for 24h, then add 10mL of ethyl acetate solvent, back-extract with water, collect the organic phase, concentrate, SephadexLH -20 column chromatography to obtain the compound of formula B as white powder.
[0047] 1 H-NMR (CDCl 3 ,400MHz)δ:1.72(3H,s,CH3-5″),1.76(3H,s,CH3-4″),2.35(3H,s,CH3CO),2.83(1H,dd,J=2.8,16.8Hz ,H-3),3.04(1H,dd,J=13.2,16.8Hz,H-3),3.27(2H,d,J=7.2Hz,H-1″),3.88(3H,s,OCH3), 5.29(1H,m,H-2″),5.47(1H,dd,J=2.8,13.2Hz,H-2),6.47(1H,s,H-8),7.18(2H,d,J=8.4 Hz, H-3', 5'), 7.52 (2H, d, J=8.4Hz, H-2', 6'), 7.70 (1H, s, H-5).
Embodiment 3
[0048] Embodiment 3: the preparation of psoralen flavone methyl ether (compound of formula C)
[0049]
[0050] Formula 3 intermediate: preparation of 2'-isopentenyloxy-4'-methoxyacetophenone:
[0051] Add 500mg of 2'-hydroxy-4'-methoxyacetophenone (compound of formula 1), 1.25g of anhydrous potassium carbonate, 15mL of acetone and 0.4mL of prenyl bromide (compound of formula 2) into the reaction flask, and make The temperature of the reaction solution was raised to reflux, and the heat preservation reaction was carried out; when the reaction was monitored by TLC (about 5 hours of reflux reaction), the reaction solution was cooled to room temperature, extracted with 30 mL of ethyl acetate and 15 mL of water, and the ethyl acetate layer was collected and carried out with saturated NaCl solution. After washing, drying over anhydrous sodium sulfate, and filtering, the filtrate was purified by silica gel column chromatography and rinsed with ethyl acetate / petroleum ether to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com