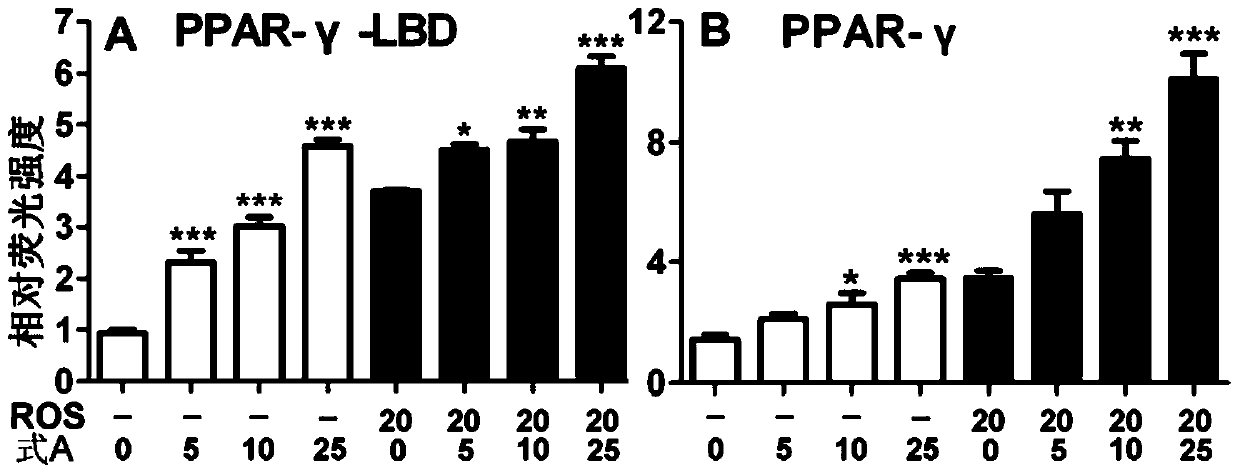
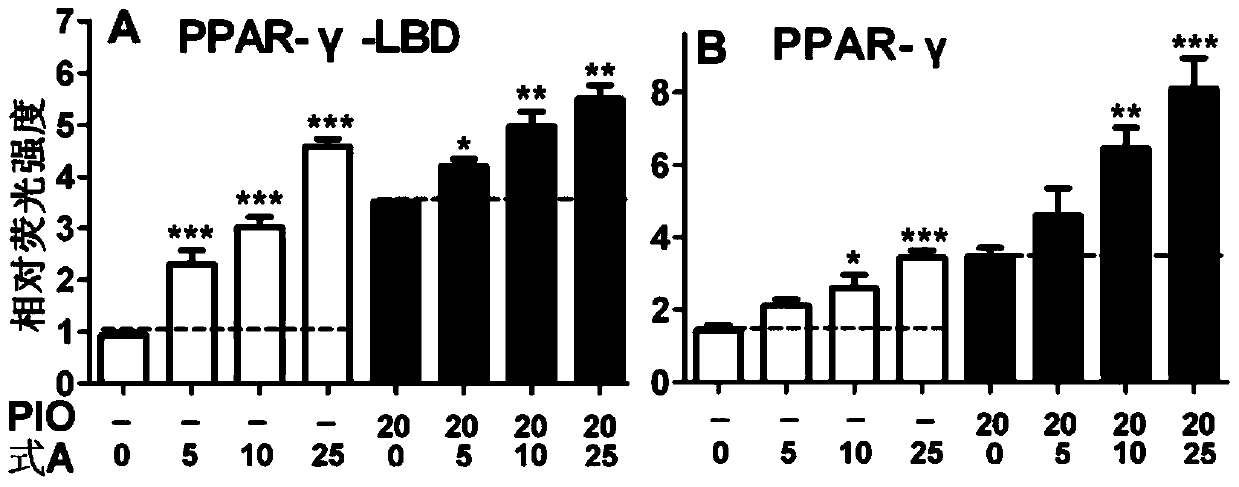
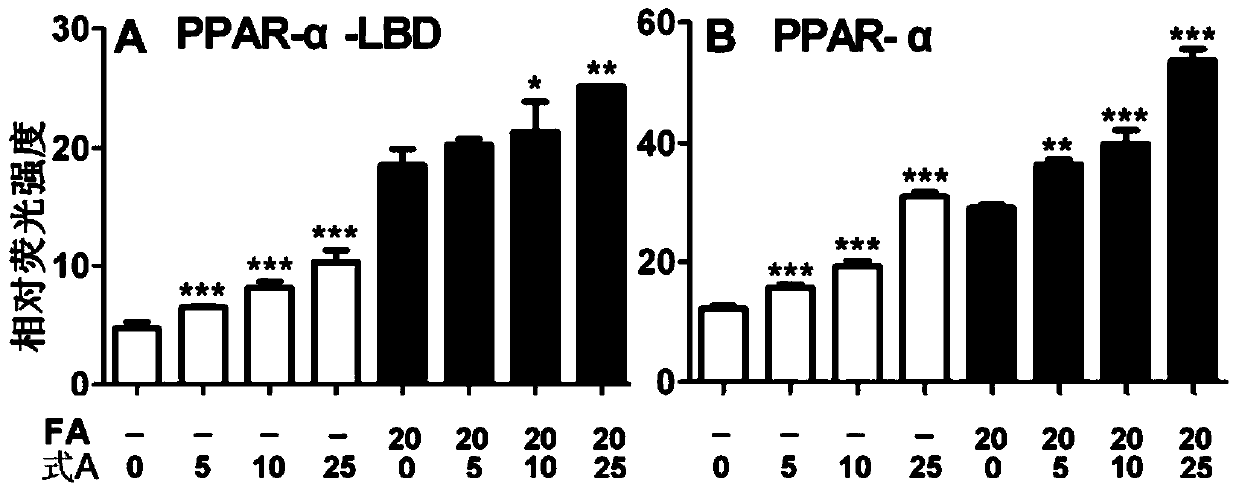
A kind of use of bakuchiol dihydroflavonoid methyl ether and analogs thereof
A technology of flavone methyl ether of psoraleae and its analogues, which is applied in the field of medicine and can solve the problems of toxic side effects, influence and limitation of clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of bakuchiol dihydroflavonoid methyl ether (compound of formula A)
[0040] 10.0kg of Psoraleae medicinal material was extracted with 8 times the volume (80L) of a 95% ethanol aqueous solution, refluxed for 2 hours each time, and refluxed for extraction 3 times in total, combined the extracts, and concentrated under reduced pressure to obtain the extract (about 800mL); 1 times the amount (800mL) of water was added to the extract to suspend, followed by extraction with petroleum ether (1000mL×3) and ethyl acetate (1000mL×3), and the ethyl acetate extract was collected; recovery under reduced pressure Silica gel column chromatography followed by ethyl acetate, followed by gradient elution with petroleum ether and ethyl acetate (10:1-1:5); Elution (9:1-1:1), then use reverse column chromatography methanol-water gradient elution (60% methanol-80% methanol), and finally purify with Sephadex LH-20 (methanol) to obtain formula A Compound, white powder. ...
Embodiment 2
[0045] Example 2: Preparation of compound of formula B
[0046] Dissolve 20 mg of bakuchiol dihydroflavonoid methyl ether [compound of formula A] in 1 mL of pyridine, then add 1 mL of acetic anhydride, leave it at room temperature for 24 h, then add 10 mL of ethyl acetate solvent, back-extract with water, collect the organic phase, concentrate, Sephadex LH-20 column chromatography, the compound of formula B is obtained as white powder.
[0047] 1 H-NMR (CDCl 3 ,400MHz)δ:1.72(3H,s,CH3-5″),1.76(3H,s,CH3-4″),2.35(3H,s,CH3CO),2.83(1H,dd,J=2.8,16.8Hz ,H-3),3.04(1H,dd,J=13.2,16.8Hz,H-3),3.27(2H,d,J=7.2Hz,H-1″),3.88(3H,s,OCH3), 5.29(1H,m,H-2″),5.47(1H,dd,J=2.8,13.2Hz,H-2),6.47(1H,s,H-8),7.18(2H,d,J=8.4 Hz, H-3', 5'), 7.52 (2H, d, J=8.4 Hz, H-2', 6'), 7.70 (1H, s, H-5).
Embodiment 3
[0048] Example 3: Preparation of bakuchiin flavonoid methyl ether (compound of formula C)
[0049]
[0050] Preparation of intermediate of formula 3: 2'-prenyloxy-4'-methoxyacetophenone:
[0051] 500 mg of 2'-hydroxy-4'-methoxyacetophenone (compound of formula 1), 1.25 g of anhydrous potassium carbonate, 15 mL of acetone and 0.4 mL of isopentenyl bromide (compound of formula 2) were added to the reaction flask to make The reaction solution was heated to reflux, and the reaction was incubated at the end of the reaction by TLC monitoring (about 5h of reflux reaction), the reaction solution was cooled to room temperature, extracted with 30 mL of ethyl acetate and 15 mL of water, the ethyl acetate layer was collected, and saturated NaCl solution was used to carry out Washed, dried over anhydrous sodium sulfate, filtered, and the filtrate was purified by silica gel column chromatography and rinsed with ethyl acetate / petroleum ether to obtain the intermediate of formula 3 as a ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com