Regadenoson injection and preparation method thereof
A technology of Reganoson and injection, which is applied in the field of Reganoson injection and its preparation, can solve the problems of reducing blood calcium ions, etc., achieve the effects of reducing irritation, improving sterility, and ensuring smooth progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
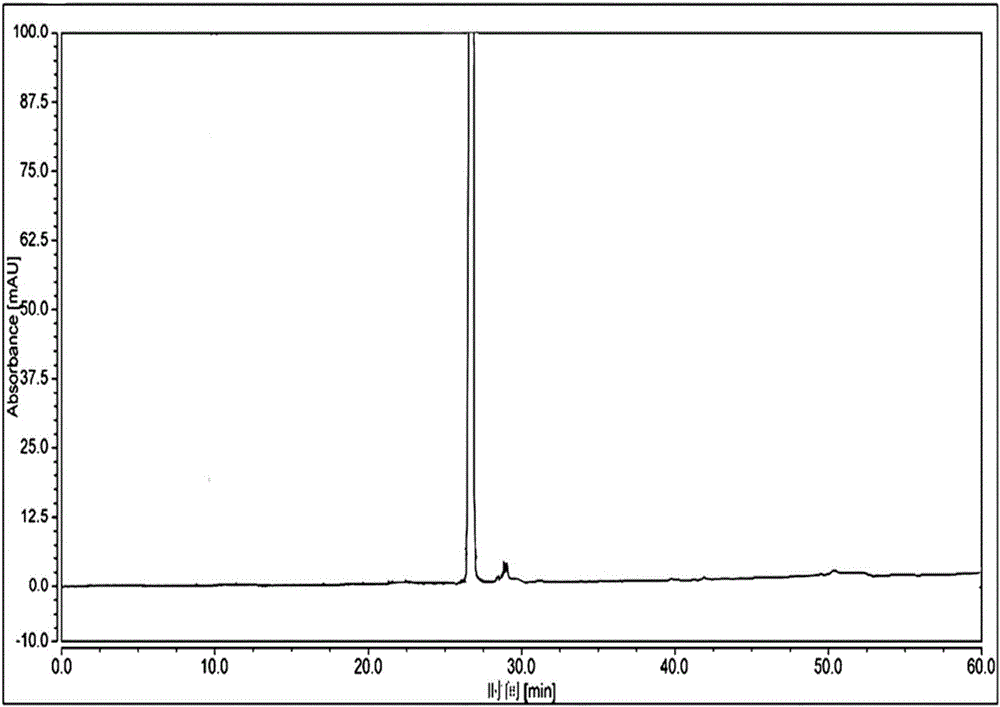
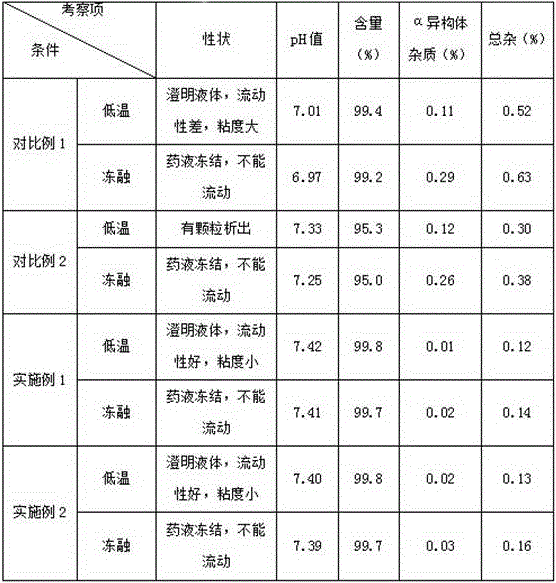
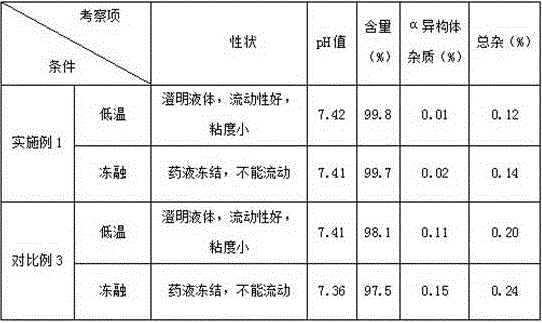
Image
Examples
Embodiment 1
[0061] Example 1 Reganoson Injection (0.4mg / 5mL), 5000 vials
[0062] Prescription: Reganoson 2g, histidine 300g, L-cysteine 50g, tris 165g, sodium chloride 225g, hydrochloric acid to adjust the pH to 7.4, water for injection to 25L.
[0063] Preparation:
[0064] (1) Under the protection of nitrogen, dissolve histidine and L-cysteine in an appropriate amount of water for injection at 65°C to make a concentrated solution with a cosolvent content of 30% (w / w), and slowly add Regano (2) Slowly lower the temperature to 45°C-50°C, add tris to dissolve, then add 70% of the prescribed amount of water for injection to dilute;
[0065] (3) Cool to 25±2°C, add sodium chloride, adjust the pH to 7.4 with hydrochloric acid, and make up the water for injection to the full amount;
[0066] (4) Use a 5000MW membrane ultrafilter to filter the medicinal solution to remove pyrogens, collect the ultrafiltrate, and then pass it through a 0.22μm polyethersulfone filter element for fine filtr...
Embodiment 2
[0069] Example 2 Reganoson Injection (0.4mg / 5mL), 5000 vials
[0070] Prescription: Reganoson 2g, histidine 400g, arginine 50g, tris 170g, sodium bisulfite 30g, sodium chloride 225g, hydrochloric acid to adjust the pH to 7.4, water for injection to 25L.
[0071] Preparation:
[0072] (1) Under the protection of nitrogen, dissolve histidine and arginine in an appropriate amount of water for injection at 65°C to make a concentrated solution with a cosolvent content of 30% (w / w), add sodium bisulfite to dissolve, and then Slowly add Reganosen, keep stirring to make it completely dissolved;
[0073] (2) Slowly lower the temperature to 45°C-50°C, add tris to dissolve, then add 70% of the prescribed amount of water for injection to dilute;
[0074] (3) Cool to 25±2°C, add sodium chloride, adjust the pH to 7.4 with hydrochloric acid, and make up the water for injection to the full amount;
[0075] (4) Use a 5000MW membrane ultrafilter to filter the medicinal solution to remove pyr...
Embodiment 3
[0078] Example 3 Reganoson Injection (0.4mg / 5mL), 5000 vials
[0079] Prescription: Regganosan 2g, histidine 400g, glycine 50g, tris 170g, sodium metabisulfite 35g, sodium chloride 225g, hydrochloric acid to adjust the pH to 7.4, water for injection to 25L.
[0080] Preparation:
[0081] (1) Under the protection of nitrogen, dissolve histidine and glycine in an appropriate amount of water for injection at 60-70°C to make a concentrated solution with a cosolvent content of 30% (w / w), add sodium metabisulfite to dissolve, and then slowly add Reginaosheng, keep warm and stir to make it completely dissolved;
[0082] (2) Slowly lower the temperature to 45°C-50°C, add tris to dissolve, then add 70% of the prescribed amount of water for injection to dilute;
[0083] (3) Cool to 25±2°C, add sodium chloride, adjust the pH to 7.4 with hydrochloric acid, and make up the water for injection to the full amount;
[0084] (4) Use a 5000MW membrane ultrafilter to filter the medicinal solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com