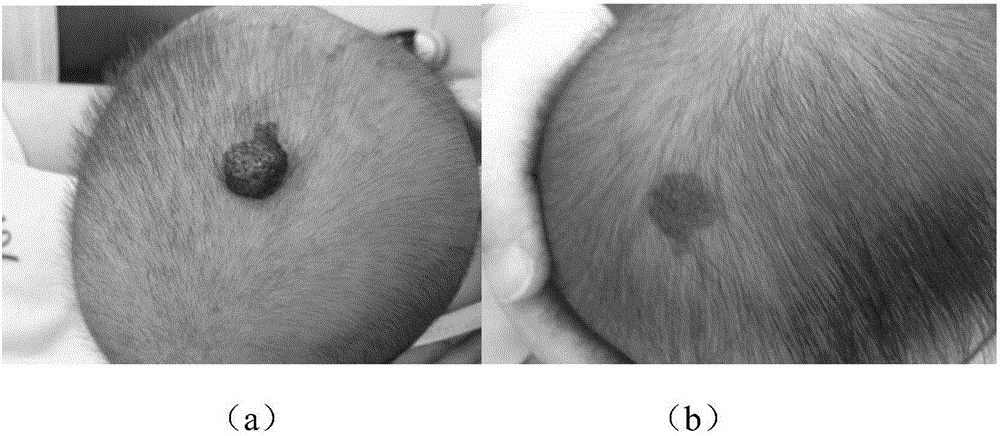
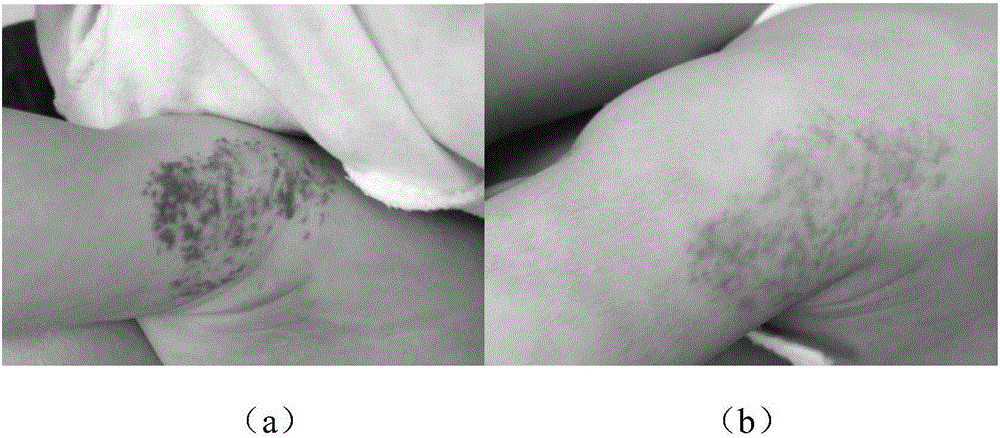
Timolol external preparation for treating hemangioma
A technology for external preparations and hemangioma, which is applied in the field of pharmaceutical preparations, can solve the problems of inflow into the eye, systemic adverse reactions, and volatile solutions, and achieves the effects of convenient application, small adverse reactions, and simple and easy process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the above-mentioned external preparation for treating hemangioma comprises the following steps:
[0027] (1) Timolol is prepared in a salt form into a timolol salt solution, and the concentration of the timolol salt solution is 0.1% to 90%;
[0028] (2) adding the active ingredient peppermint oil of formula quantity in step (1) gained timolol salt solution;
[0029] (3) Add an appropriate amount of preservative, humectant, antioxidant to the solution obtained in step (2), and stir evenly;
[0030] (4) disperse the solution obtained in step (3) in the gel matrix according to the weight ratio of 1:1 to 1:10, and swell to obtain the timolol gel;
[0031] The solution obtained in step (3) is dispersed in the ointment base according to the weight ratio of 2.5:1 to 3:1 to obtain timolol ointment.
[0032] Wherein, the preservative is selected from one of ethylparaben, butylparaben, chlorobutanol, sodium benzoate and sorbic acid.
[0033] The humec...
Embodiment 1
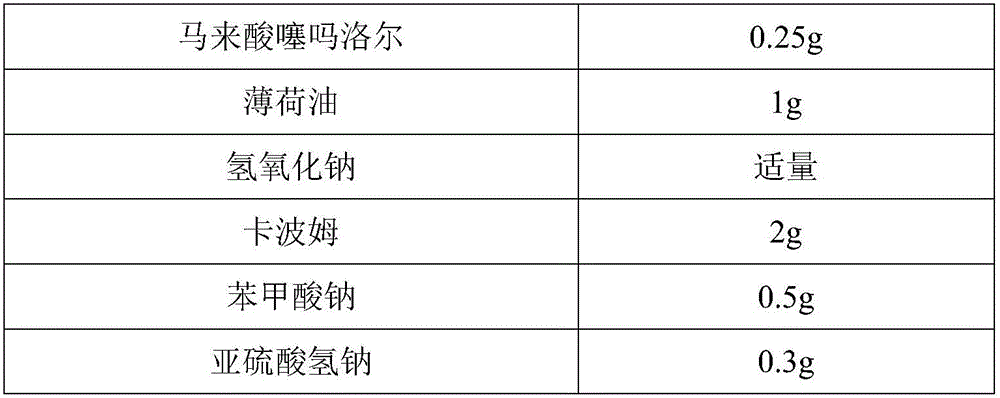
[0038] Prescription (percentage by weight):
[0039]
[0040]
[0041] Preparation Process:
[0042] (1) get timolol maleate and sodium bisulfite of prescription quantity, accurately weighed, be dissolved in 10ml water, make aqueous phase;
[0043] (2) Add carbomer to the remaining water and stir, and add sodium hydroxide as a pH regulator to adjust the pH to 7-8 to make it fully swell to obtain a gel matrix;
[0044] (3) Add the aqueous phase obtained in step (1) into the gel matrix, and add peppermint oil, sodium benzoate, propylene glycol, etc., to obtain an external gel for treating hemangioma.
Embodiment 2
[0046] Prescription (percentage by weight):
[0047] timolol maleate
0.5g
peppermint
1g
2.5g
0.5g
0.3g
glycerin
2g
water
Add to 100g
[0048] Preparation Process:
[0049] (1) get timolol maleate and sodium bisulfite of prescription quantity, accurately weighed, be dissolved in 20ml water;
[0050] (2) adding sodium alginate to the remaining water, stirring overnight to make it fully swell to obtain a gel matrix;
[0051] The aqueous phase obtained in the step (1) is added into the gel matrix, and peppermint oil, sorbic acid, glycerin and the like are added to obtain the external gel for treating hemangioma.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com