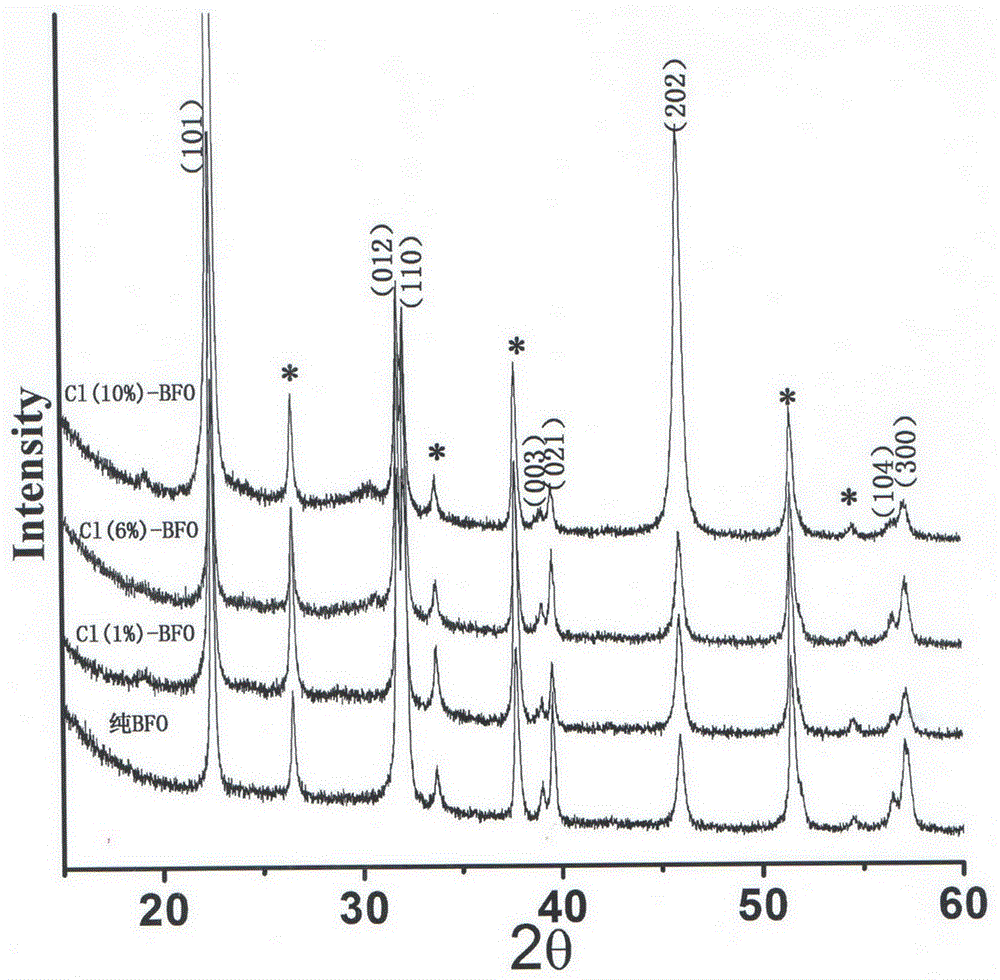
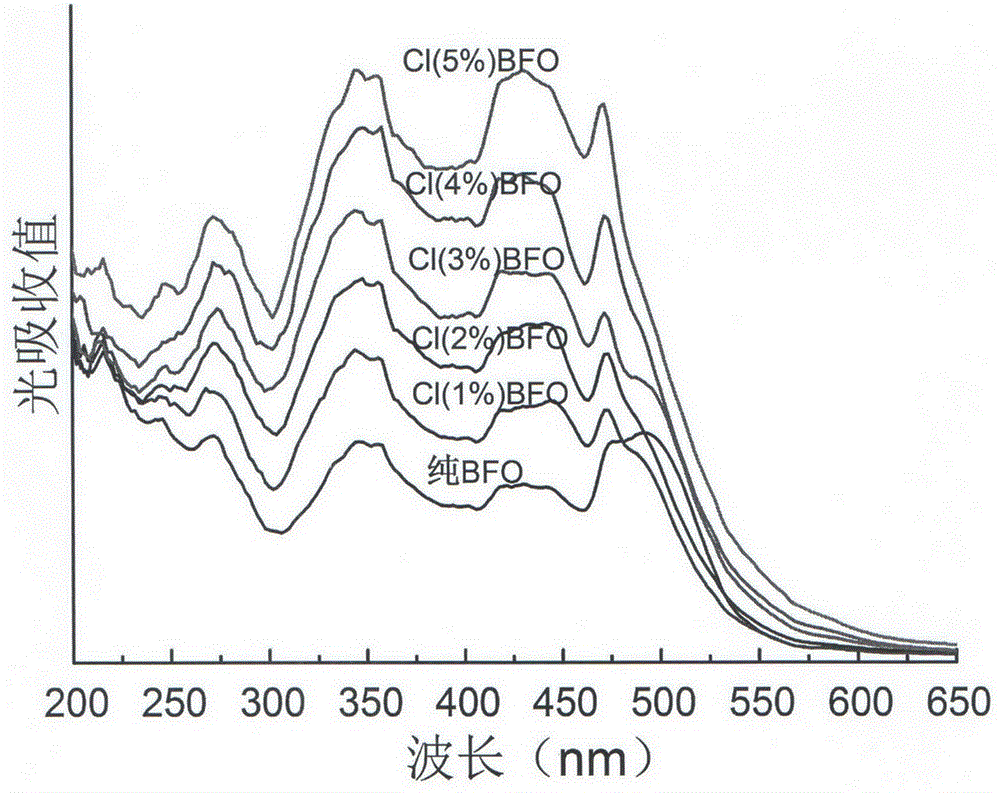
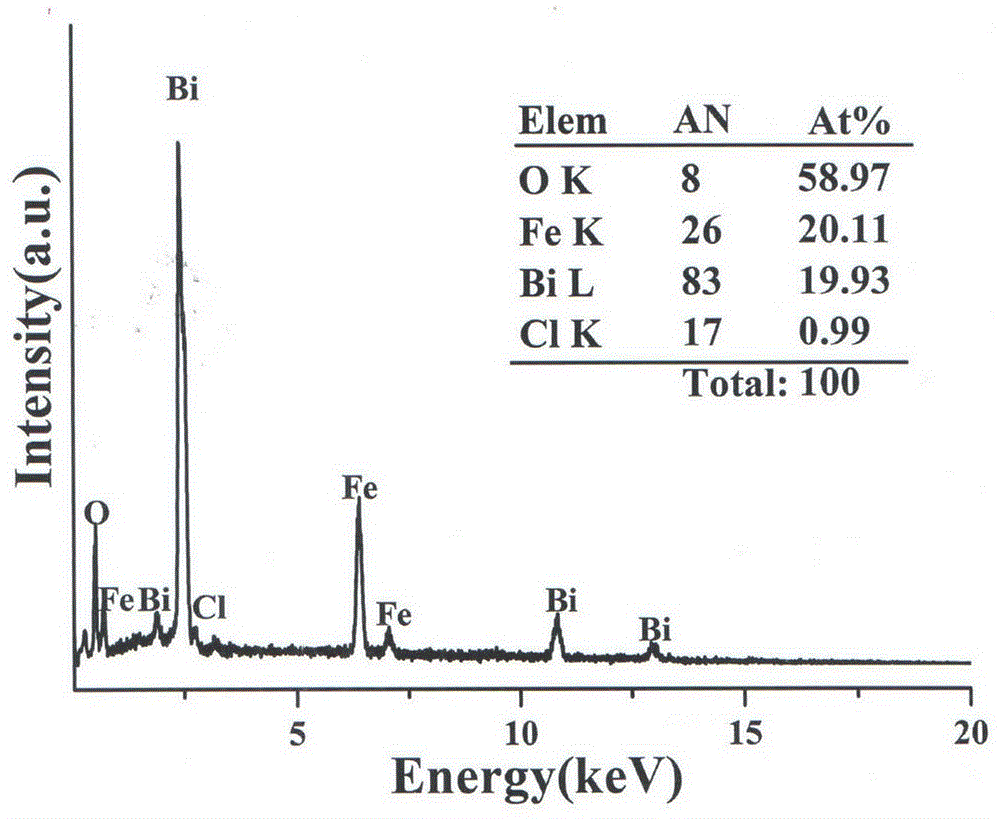
Preparation method of chlorine-doped bismuth ferrite photoelectric film
A photoelectric thin film, bismuth ferrite technology, applied in the manufacture of circuits, electrical components, final products, etc., can solve the problems of inability to remove unreacted raw materials and by-products with concentrated nitric acid, unsuitable thin film materials, etc., and achieve easy control of thin film thickness, The doping concentration is easy to control and the effect of increasing the light absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] a, ferric trichloride, ferric nitrate, bismuth nitrate are mixed in a molar ratio of 0.01:1:1.05 (that is, ferric trichloride 0.0033g, ferric nitrate 0.4847g, bismuth nitrate 0.8295g), according to the content of bismuth ions in the solute The volume ratio of the solvent to the solvent ethylene glycol methyl ether is 0.21mol / L, and then mixed with 10ml of the solvent ethylene glycol methyl ether, heated and stirred at 50°C for 30min, then cooled to room temperature, added 10ml of glacial acetic acid, and stirred at room temperature for 3h. Stand and age for 12h, filter to obtain the clarified liquid;
[0021] b. Take 40 μl of the clarified solution obtained in step a and drop it on the 2 On the fluorine-doped tin oxide transparent conductive glass substrate, use a spin coater to spin coat at a speed of 3000r / min for 20 seconds;
[0022] c. Place the sample obtained in step b in a tube furnace, heat it at a temperature of 300° C. for 5 minutes, and cool it to room tempe...
Embodiment 2
[0026] a. Ferric trichloride, ferric nitrate, and bismuth nitrate are mixed in a molar ratio of 0.02:1:1.05 (that is, ferric trichloride 0.0129g, ferric nitrate 0.9694g, bismuth nitrate 1.6589g), according to the content of bismuth ions in the solute The volume ratio of volume to solvent is 0.42mol / L, then mixed with 10ml of solvent ethylene glycol methyl ether, heated and stirred at 50°C for 10min, then cooled to room temperature, added 10ml of glacial acetic acid solvent, stirred at room temperature for 1h, and left to age 12h, filter to obtain clarified liquid;
[0027] b. Take 40 μl of the clarified solution obtained in step a and drop it on the 2 On the silicon substrate, use the spin coater to spin coat for 20 seconds under the rotating speed of 2000r / min;
[0028] c. Place the sample obtained in step b in a tube furnace, heat it at 200° C. for 5 minutes, and cool it to room temperature in air;
[0029] d. On the sample obtained in step c, repeat step b and step c 10 t...
Embodiment 3
[0032] a, ferric trichloride, ferric nitrate, bismuth nitrate are mixed in a molar ratio of 0.02:1:1.05 (i.e. ferric trichloride 0.0194g, ferric nitrate 1.4541g, bismuth nitrate 2.4884g), according to the content of bismuth ions in the solute The volume ratio of volume to solvent is 0.63mol / L, then mixed with 10ml of solvent ethylene glycol methyl ether, heated and stirred at 50°C for 20min, then cooled to room temperature, added 10ml of glacial acetic acid solvent, stirred at room temperature for 6h, and left to age 12h, filter to obtain clarified liquid;
[0033] b. Take 40 μl of the clarified solution obtained in step a and drop it on the 2 On the indium tin oxide transparent conductive glass substrate, use a spin coater to spin coat at a speed of 4000r / min for 20 seconds;
[0034] c. Place the sample obtained in step b in a tube furnace, heat it at a temperature of 300° C. for 5 minutes, and cool it to room temperature in air;
[0035] d. On the sample obtained in step c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com