Method for preparing azodicarbonamide by oxygen oxidation
An azodicarbonamide and oxygen oxidation technology, applied in the direction of organic chemistry, can solve the problems of high equipment requirements, corrosion equipment, and high equipment requirements, and achieve the effects of new preparation technology, less product impurities, and uniform particle size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
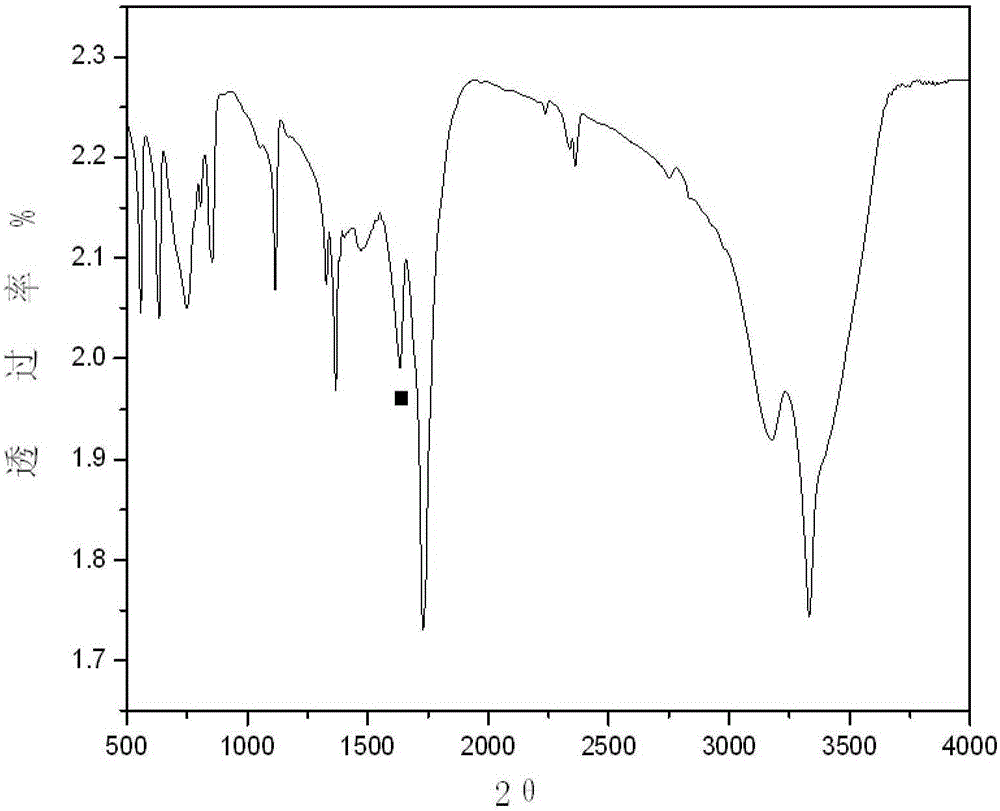
[0014] Embodiment 1: choose cobalt-manganese catalyst 15g that is made up of cobalt chloride and manganese nitrate, cobalt-manganese ion ratio is 1:1 in the catalyst, take biurea 100g, add 500ml deionized water to dissolve and form solution, solution is heated to 70 ℃, and continue to feed oxygen to keep the pH of the solution at 7. After 6 hours of reaction, the reaction is terminated. After filtration, the filter cake is washed three times with 100ml distilled water, and vacuum-dried at 90°C to obtain 91.5g of the product. After IR, IH-NMR and ESI-MS analysis confirmed that its structure is azodicarbonamide with a yield of 93.1% and a content of more than 99.5%. The detection method refers to HG2097-2008.
Embodiment 2
[0015] Example 2: Select 20g of a cobalt-manganese catalyst composed of cobalt sulfate and manganese sulfate, the ratio of cobalt-manganese ions in the catalyst is 2:1, take 100g of biurea, add 500ml of deionized water to dissolve and form a solution, and heat the solution to 70°C , and continue to feed oxygen to keep the pH of the solution at 7. After 6 hours of reaction, the reaction is terminated. After filtration, the filter cake is washed three times with 100ml distilled water, and vacuum-dried at 90°C to obtain 93.4g of the product. After IR, IH-NMR and ESI -MS analysis confirms that its structure is azodicarbonamide, the yield rate is 95.1%, and the content is greater than 99.5%. The detection method refers to HG2097-2008.
Embodiment 3
[0016] Example 3: Select 15g of cobalt-manganese catalyst composed of cobalt acetate and manganese acetate, the ratio of cobalt-manganese ions in the catalyst is 3:1, take 100g of biurea, add 500ml of deionized water to dissolve and form a solution, and heat the solution to 80°C , and continue to feed oxygen to keep the pH of the solution at 7. After 7 hours of reaction, the reaction is terminated. After filtration, the filter cake is washed three times with 100ml distilled water, and vacuum-dried at 90°C to obtain 91.5g of the product. After IR, IH-NMR and ESI -MS analysis confirms that its structure is azodicarbonamide, the yield rate is 93.1%, and the content is greater than 99.5%. The detection method refers to HG2097-2008.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com