A kind of stilbene derivative and preparation method thereof
A technology of stilbenes and derivatives, which is applied in the fields of drug combination, organic chemistry, and nervous system diseases, etc., can solve the problem of large dosage, inability to effectively pass through the blood-brain barrier, and failure to meet the optimal physical and chemical properties of breaking through the blood-brain barrier And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
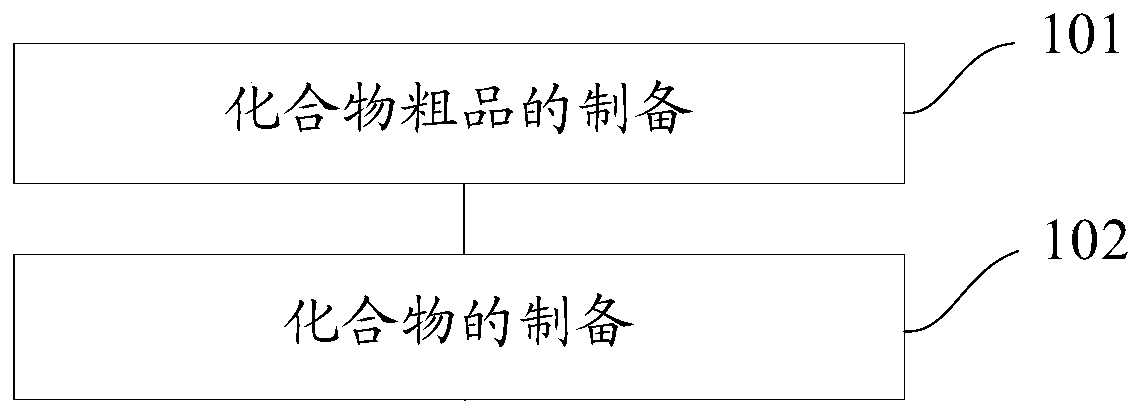
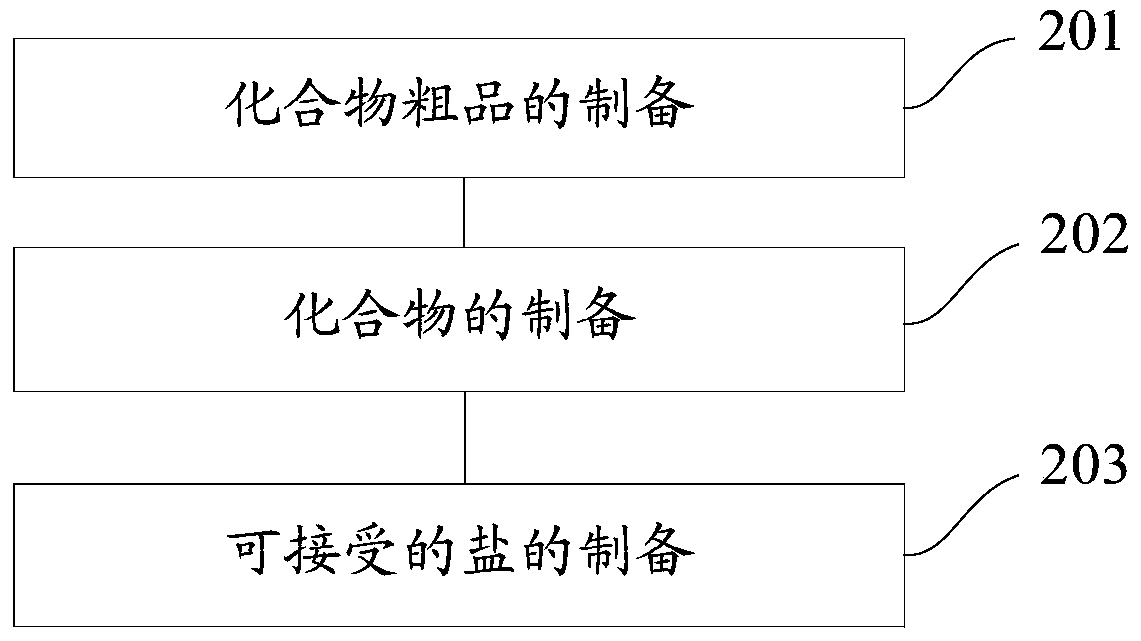
[0144] The present invention also provides a method for preparing a novel stilbene derivative. Specifically, the method for preparing a compound having the general formula I or the general formula II includes:
[0145] Preparation of the crude compound: dissolve the reaction substrate in dichloromethane, add a catalyst to obtain a mixture, add dropwise an acylating agent or a treated acylating agent to the mixture and stir until the reaction ends, and evaporate the two to dryness. Methyl chloride to obtain the crude product of the compound, and the reaction substrate is E-4'-amino-3,4-methylenedioxy-stilbene or E-3,4-methylenedioxy-3' -Fluoro-4'-amino-diphenylethylene;
[0146] Preparation of compound: to the crude compound was added saturated NaHCO 3 solution, additionally add dichloromethane for extraction, obtain the organic phase of the lower layer, wash the organic phase with distilled water and saturated brine successively, and perform rotary evaporation, concentration ...
example 1
[0173] Example 1: The reaction formula for preparing E-4-amino-3',4'-methylenedioxy-diphenylethylene (WS-4) is:
[0174]
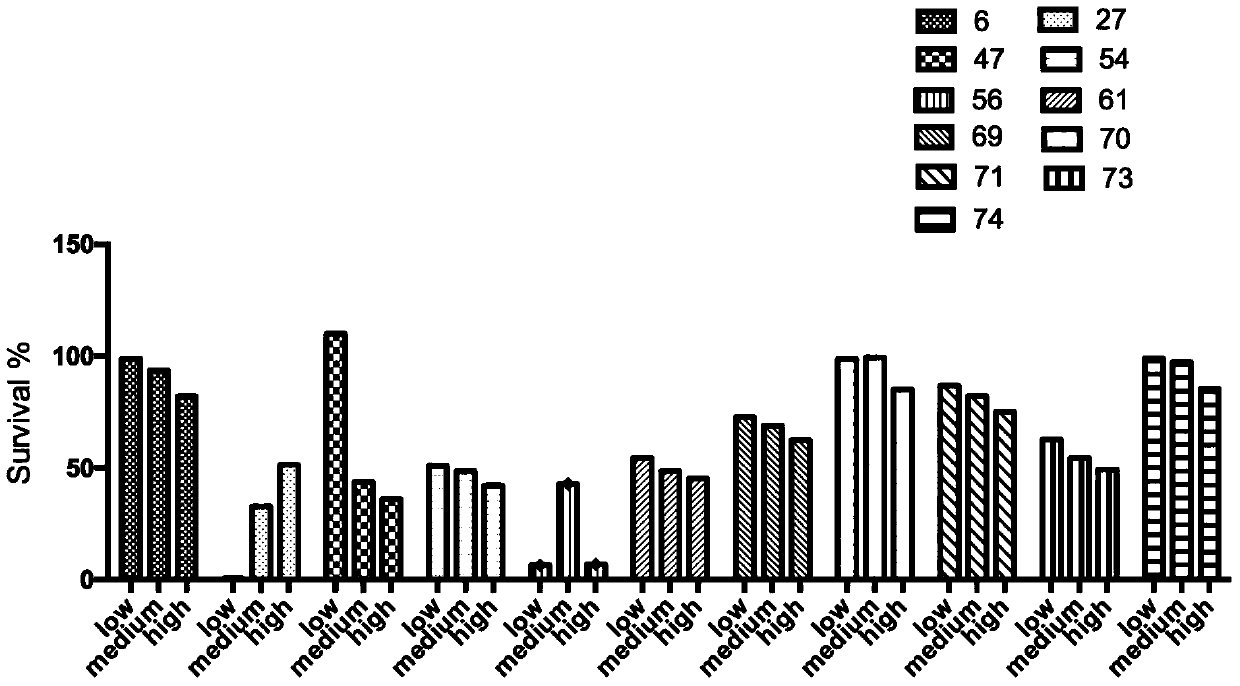
[0175] The specific steps may include: 3,4-methylenedioxy-iodobenzene (6.63mmol, 1.644g) was dissolved in DMF (60mL) solution, tetrabutylammonium bromide (3.33mmol, 1.10g) was added, acetic acid Sodium (3.57mmol, 0.586g), palladium acetate (0.11mmol, 0.025g) and 4-nitro-styrene (2.44mmol, 0.365g). The reaction system was replaced with argon 5 times, and stirred at 80° C. for 5 h under the protection of argon. After the reaction was completed, distilled water (60 mL) was added to terminate the reaction, extracted with ethyl acetate (75 mL), the upper organic phase was washed with water (60 mL), and then saturated brine (75 mL), concentrated by rotary evaporation, and dried to obtain a solid. Dissolve the solid in absolute ethanol (45 mL), add stannous chloride (33.15 mmol, 6.261 g), and stir at reflux for 4 h. After the reaction, the ethanol was evapor...
example 2
[0177] Example 2: The reaction formula for preparing E-4-acetylamino-3',4'-methylenedioxy-diphenylethylene (WS-6) is:
[0178]
[0179] The specific steps may include: adding compound WS-4 (2.22mmol, 0.532g) to a 100mL round bottom flask, stirring and dissolving with anhydrous dichloromethane (20mL), adding pyridine (6.66mmol, 0.518g), DMAP (0.22mmol ,0.027g), cooled in an ice-water bath to below 0°C, slowly added acetic anhydride (6.66mmol, 0.626ml) dropwise, keeping the temperature below 0°C during the dropwise addition, and stirred in an ice-water bath for 0.5h. After the reaction, add saturated NaHCO 3 solution (25 mL), stirred for 0.5 h. An additional 10 mL of dichloromethane was added for extraction, and the lower organic phase was washed with water (20 mL) and then with saturated brine (25 mL), concentrated by rotary evaporation, and dried to obtain a solid. After column chromatography (200-400 mesh silica gel, mobile phase V (dichloromethane): V (methanol) = 10:0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com