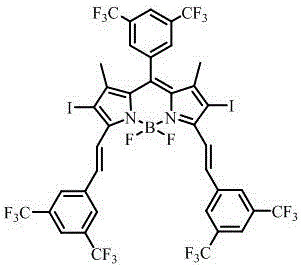
BODIPY derivative containing six trifluoromethyl groups and preparation and application of BODIPY derivative
A technology of trifluoromethyl group and fluoroboron dipyrrole, which is applied in the field of synthesis and design of anticancer photosensitizers for photodynamic therapy, can solve problems such as weak tissue penetration ability, uncertain composition, skin phototoxicity, etc. Achieve the effect of not easy skin phototoxicity, low cost, and improving physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the fluoroboron dipyrrole derivative containing six trifluoromethyl groups comprises the following steps:
[0031] (1) Compound , Add it to 100 mL of anhydrous dichloromethane at a molar ratio of 1:2.5, then add a drop of trifluoroacetic acid (TFA), and stir overnight at room temperature under nitrogen protection; Benzoquinone (DDQ) was dissolved in 150 mL of dichloromethane, and then added to the reaction solution, and the resulting mixture was stirred at room temperature for 4 h; triethylamine (Et 3 N, 12 mL) and boron trifluoride diethyl ether (BF 3 ·Et 2 O, 12 mL) were slowly added dropwise to the above mixture, and stirred overnight at 0 °C; 3 The solution and water were washed twice, and the combined organic layers were washed with anhydrous Na 2 SO 4 After drying, it was spin-dried under reduced pressure; using dichloromethane-petroleum ether (1:2, v / v) as eluent, purified by silica gel column chromatography to obtain compound 1 ...
Embodiment 1
[0036] A preparation method of fluoroboron dipyrrole derivatives containing six trifluoromethyl groups, the specific steps are:
[0037] (1) Compound (1.40 g, 5.78 mmol), (1.22 g, 12.80 mmol) was added to 100 mL of anhydrous dichloromethane, then 0.05 mL of trifluoroacetic acid (TFA) was added, and stirred overnight at room temperature under nitrogen protection; 2,3-dichloro-5,6-dicyano p-Benzoquinone (DDQ) (1.32 g, 5.80 mmol) was dissolved in 150 mL of dichloromethane, then added to the reaction solution, and the resulting mixture was stirred at room temperature for 4 h; triethylamine (Et 3 N, 12 mL) and boron trifluoride diethyl ether (BF 3 ·Et 2 O, 12 mL) were slowly added dropwise to the above mixture, and stirred overnight at 0 °C; 3 The solution and water were washed twice, and the combined organic layers were washed with anhydrous Na 2 SO 4 After drying, it was spin-dried under reduced pressure; using dichloromethane-petroleum ether (1:2, v / v) as eluent, purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com