A process for removing urea from water
A urea, hydration technology, applied in chemical instruments and methods, water pollutants, natural water treatment, etc., can solve the problems of insufficient reaction, urea dispersion, high capital and operating costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
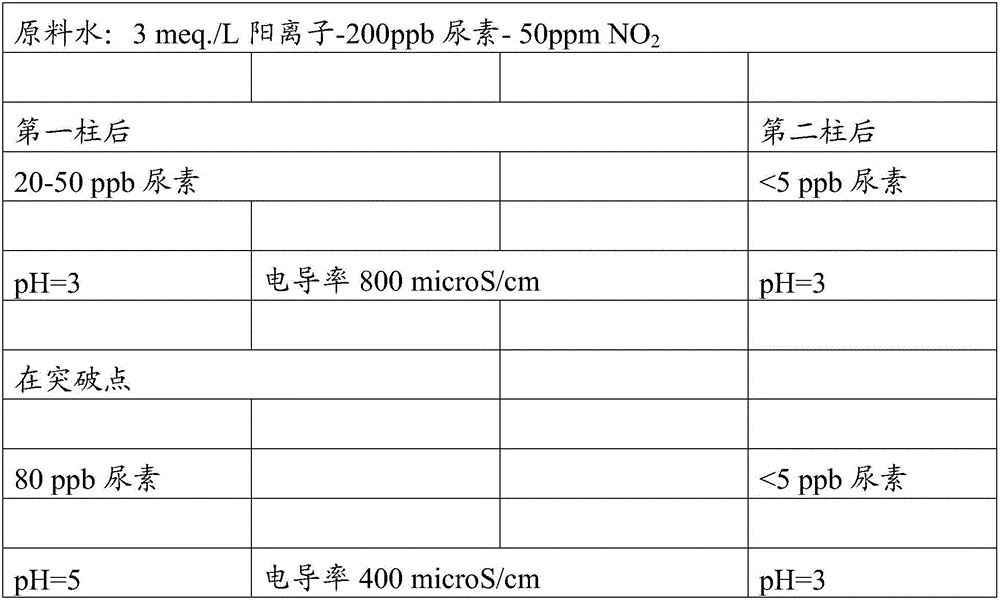
[0009] More specifically, the improved method of the present invention includes one or two cation exchange columns connected in series, each packed with a strongly acidic cation exchange resin type R-SO 3 -H+ (polystyrene cross-linked with DVB). The ion exchange resin is loaded with cations (calcium, magnesium, sodium, potassium, ammonium, etc.) derived from the raw water and replaced with hydronium ions (H+).
[0010] Before the method of the present invention enters one or more cation exchange containers, or before entering each container, add nitrite ion (NO 2 -), which as NaNO 2 (sodium nitrite) salt, nitrous acid (HNO 2 ) or NO 2 gas. NO 2 The molar ratio to urea should be 50 to 1500, ideally 100 to 700. The reaction that takes place on the strong anion exchange resin is:
[0011] CO(NH 2 ) 2 +HNO 2 +H + —>N 2 +CO 2 +NH 4 + +H 2 o
[0012] Ammonium (NH 4 + ) was captured on the ion exchange resin. The produced gas CO 2 and N 2 Soluble in water under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com