A method and system for continuous production of 5-fluoro-2-nitrophenol
A technology of nitrophenol and difluoronitrobenzene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high energy consumption, high cost, high risk factor, etc., and achieve easy industrial production, The effect of reducing side effects and simplifying the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
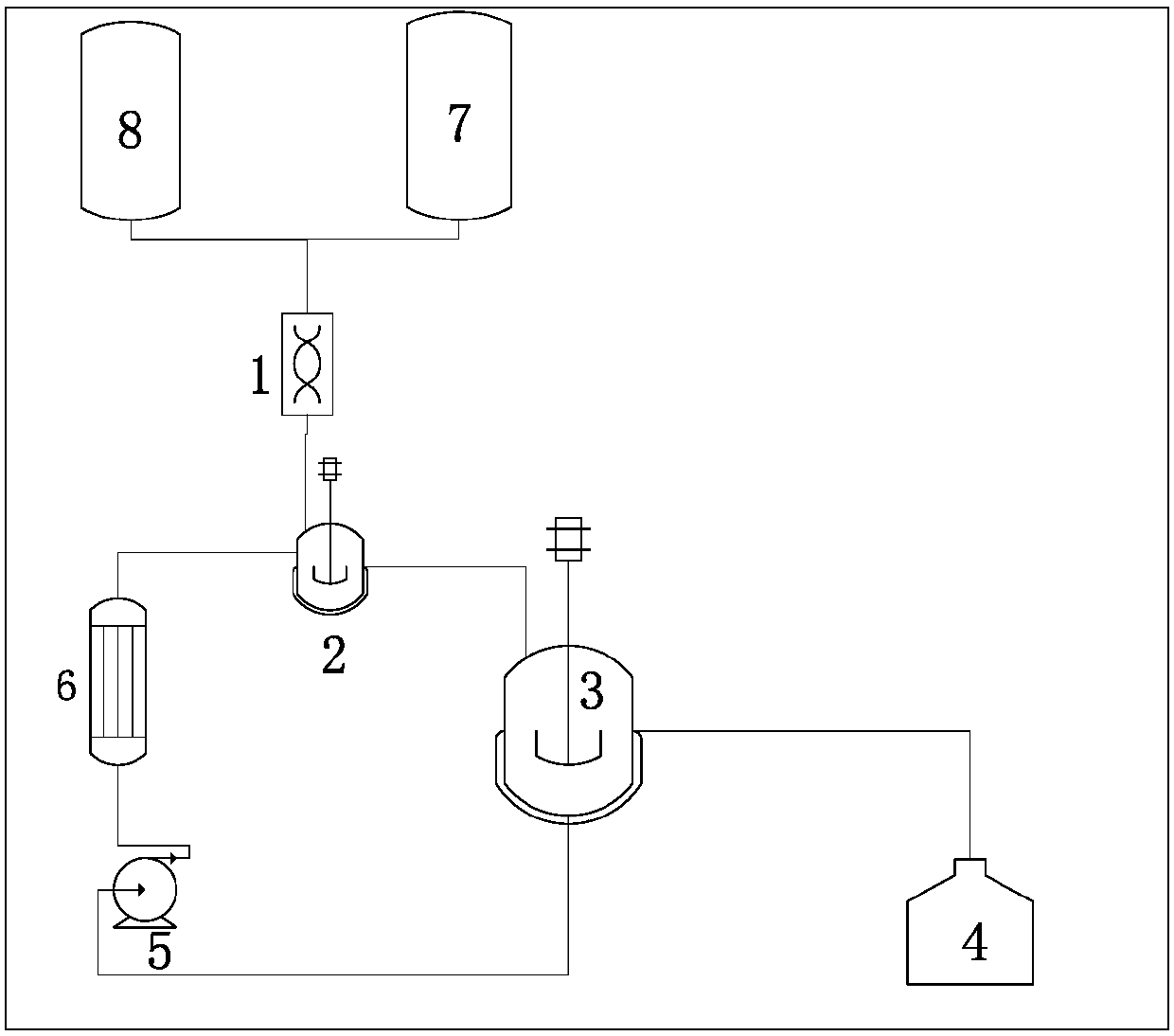
[0049] Add 200kg of 5-fluoro-2-nitrophenate potassium into the hydrolysis kettle, raise the temperature to 50-55°C, keep it warm for 1 hour, and then cool down to 45°C for use. Prepare 23.6% potassium hydroxide solution for use. In the continuous system, 2,4-difluoronitrobenzene, potassium hydroxide solution, and hydrolysis tank back-mixed liquid are added in a molar ratio of 1:2.1:17 to the hydrolysis strong mixing zone at the same time, and the linear velocity of the strong mixing zone is 50m / s, the temperature in the strong mixing zone is 50-55°C, the hydrolysis reaction of 2,4-difluoronitrobenzene and potassium hydroxide solution produces potassium phenate, and after heat preservation at 50-55°C, part of it is pumped into the strong mixing zone for hydrolysis , part of which is subjected to acidification treatment and separation treatment after cooling. The phenolic water condensed from the vapor phase of the hydrolysis tank enters the phenolic water storage tank. After ...
Embodiment 2
[0051] Add 200kg of 5-fluoro-2-nitrophenol potassium into the hydrolysis kettle, raise the temperature to 50-55°C, keep it warm for 1 hour, and then cool down to 40°C for use. Prepare 23.6% potassium hydroxide solution for use. In the continuous system, 2,4-difluoronitrobenzene, potassium hydroxide solution, and hydrolysis tank back-mixed liquid are added in a molar ratio of 1:2.2:13 to the hydrolysis strong mixing zone at the same time, and the linear velocity of the strong mixing zone in the strong mixing zone 50m / s, the temperature in the strong mixing zone is 50-55°C, the hydrolysis reaction of 2,4-difluoronitrobenzene and potassium hydroxide solution produces potassium phenate, and after heat preservation at 50-55°C, part of it is pumped into the strong mixing zone for hydrolysis , part of which is subjected to acidification treatment and separation treatment after cooling. The phenolic water condensed from the vapor phase of the hydrolysis tank enters the phenolic water...
Embodiment 3
[0053] Add 200kg of 5-fluoro-2-nitrophenate potassium into the hydrolysis kettle, raise the temperature to 50-55°C, keep it warm for 1 hour, and then cool down to 35°C for use. Prepare 23.6% potassium hydroxide solution for use. In the continuous system, 2,4-difluoronitrobenzene, potassium hydroxide solution, and hydrolysis tank back-mixed liquid are added in a molar ratio of 1:2.3:16 to the hydrolysis strong mixing zone at the same time, and the linear velocity of the strong mixing zone in the strong mixing zone 50m / s, the temperature in the strong mixing zone is 50-55°C, the hydrolysis reaction of 2,4-difluoronitrobenzene and potassium hydroxide solution produces potassium phenate, and after heat preservation at 50-55°C, part of it is pumped into the strong mixing zone for hydrolysis , part of which is subjected to acidification treatment and separation treatment after cooling. The phenolic water condensed from the vapor phase of the hydrolysis tank enters the phenolic wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com